Abstract
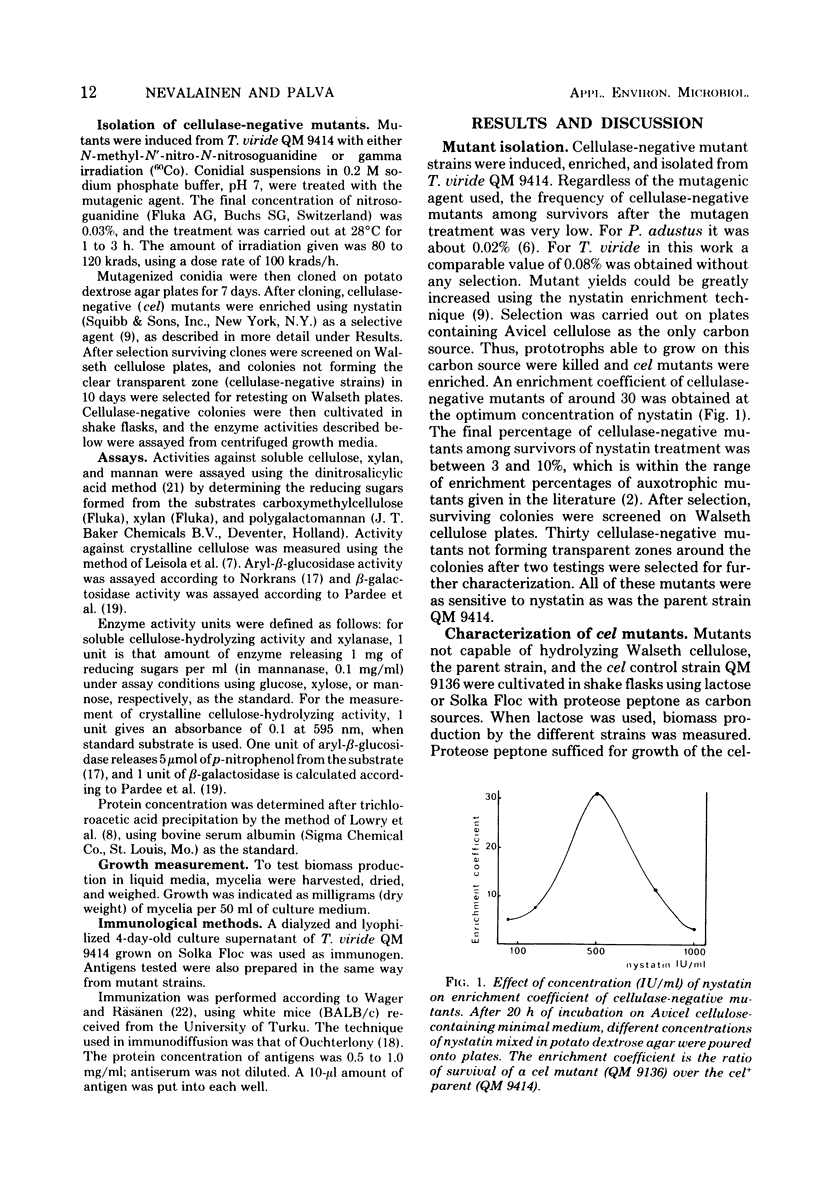
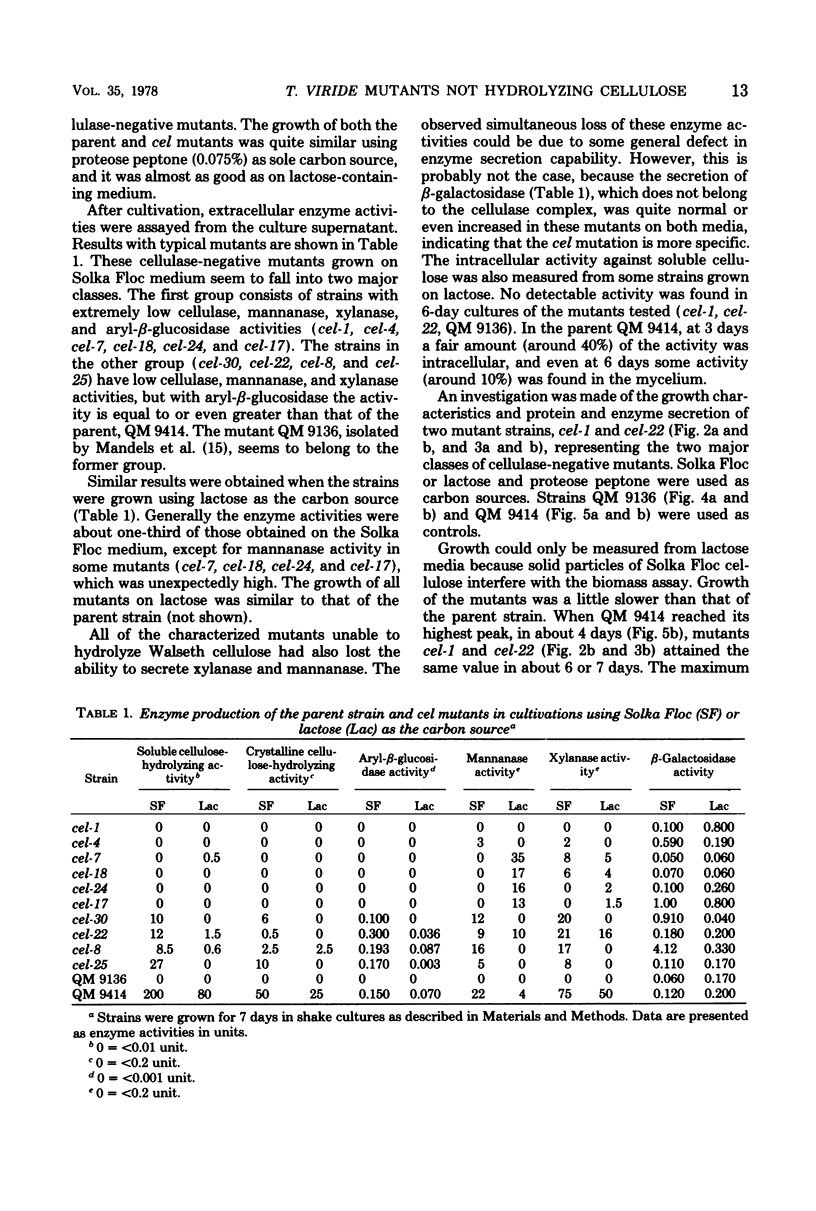
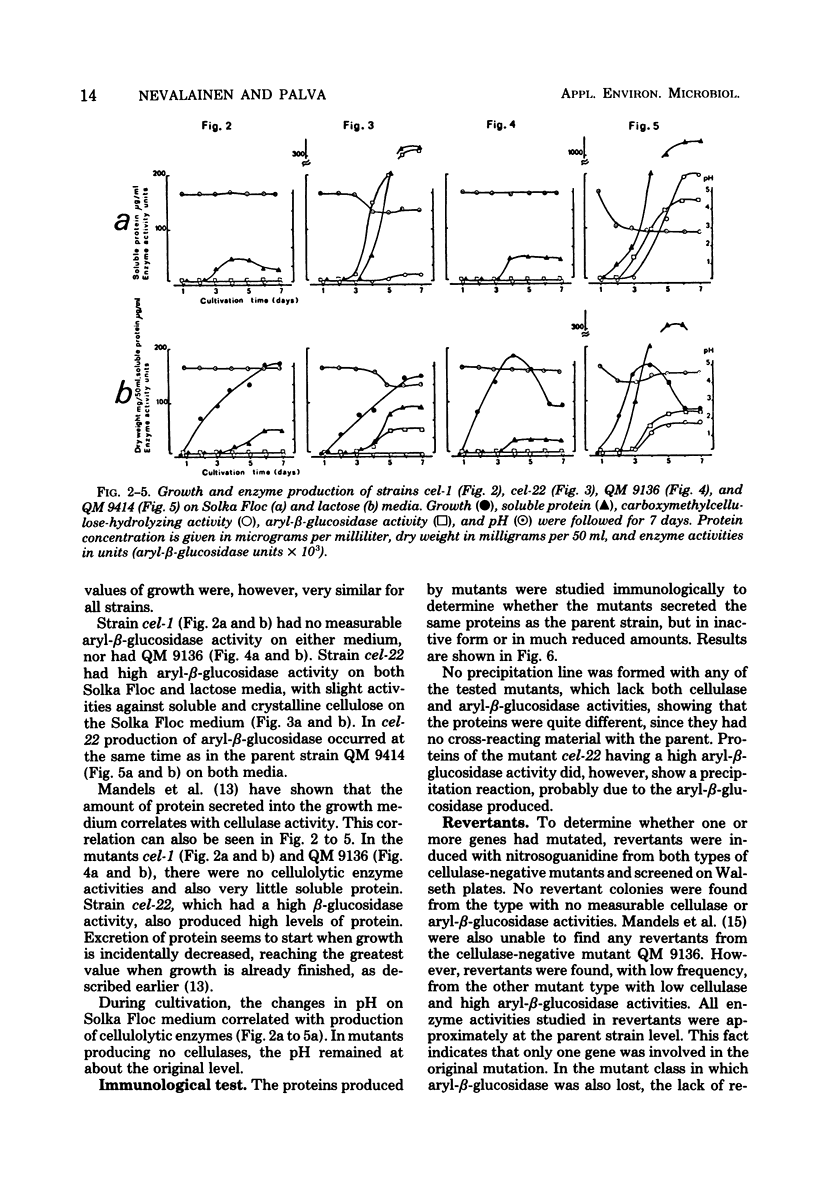
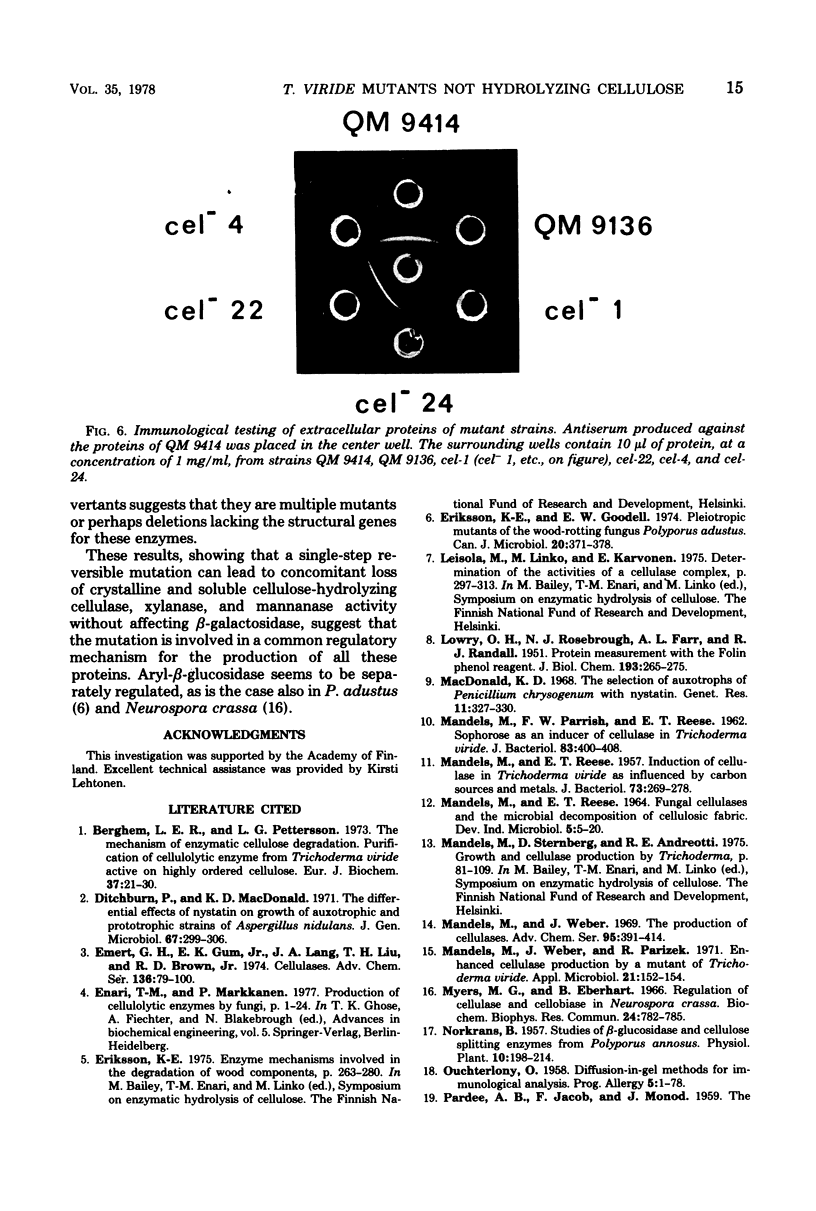
Mutant strains not producing cellulases were induced and isolated from the cellulolytic fungus Trichoderma viride. Enrichment of mutants was carried out with the aid of nystatin selection. Mutants were shown to lack the ability to hydrolyze both soluble and crystalline cellulose. Mannanase and xylanase activities were also absent, indicating a common regulation for all these enzymes in T. viride. In some strains aryl-beta-glucosidase activity was also missing. Mutants grew normally, but the amount of proteins secreted into the medium was very low, and in most cases these proteins were qualitatively different from the proteins of the parent strain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghem L. E., Pettersson L. G. The mechanism of enzymatic cellulose degradation. Purification of a cellulolytic enzyme from Trichoderma viride active on highly ordered cellulose. Eur J Biochem. 1973 Aug 1;37(1):21–30. doi: 10.1111/j.1432-1033.1973.tb02952.x. [DOI] [PubMed] [Google Scholar]
- Ditchburn P., Macdonald K. D. The differential effects of nystatin on growth of auxotrophic and prototrophic strains of Aspergillus nidulans. J Gen Microbiol. 1971 Aug;67(3):299–306. doi: 10.1099/00221287-67-3-299. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Goodell E. W. Pleiotropic mutants of the wood-rotting fungus Polyporus adustus lacking cellulase, mannanase, and xylanase. Can J Microbiol. 1974 Mar;20(3):371–378. doi: 10.1139/m74-057. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELS M., PARRISH F. W., REESE E. T. Sophorose as an inducer of cellulase in Trichoderma viride. J Bacteriol. 1962 Feb;83:400–408. doi: 10.1128/jb.83.2.400-408.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELS M., REESE E. T. Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol. 1957 Feb;73(2):269–278. doi: 10.1128/jb.73.2.269-278.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K. D. The selection of auxotrophs of Penicillium chrysognystatin. Genet Res. 1968 Jun;11(3):327–330. doi: 10.1017/s0016672300011514. [DOI] [PubMed] [Google Scholar]
- Mandels M., Weber J., Parizek R. Enhanced cellulase production by a mutant of Trichoderma viride. Appl Microbiol. 1971 Jan;21(1):152–154. doi: 10.1128/am.21.1.152-154.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Eberhart B. Regulation of cellulase and cellobiase in Neurospora crassa. Biochem Biophys Res Commun. 1966 Sep 8;24(5):782–785. doi: 10.1016/0006-291x(66)90394-9. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Selby K., Maitland C. C. The cellulase of Trichoderma viride. Separation of the components involved in the solubilization of cotton. Biochem J. 1967 Sep;104(3):716–724. doi: 10.1042/bj1040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager O., Räsänen J. A. Mouse immune ascites in immunoelectrophoresis. Ann Med Exp Biol Fenn. 1967;45(2):170–173. [PubMed] [Google Scholar]



