Abstract
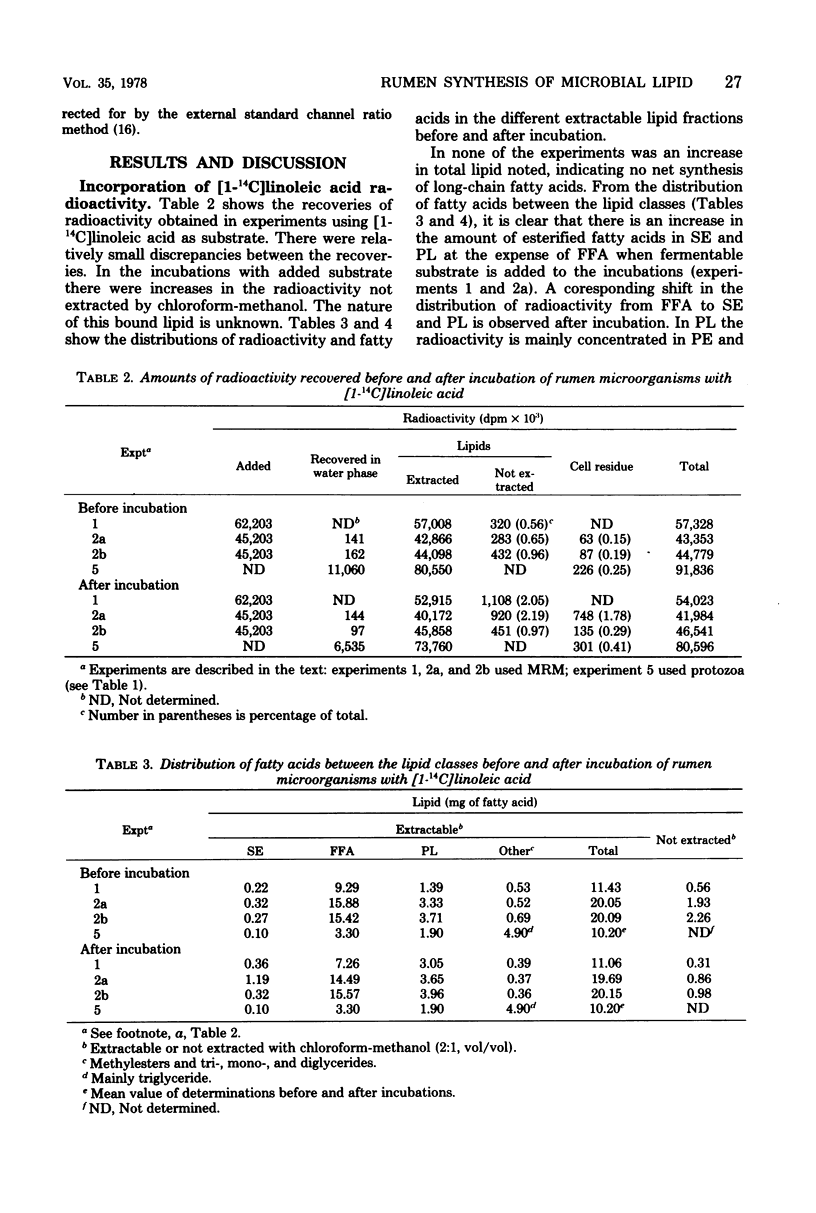
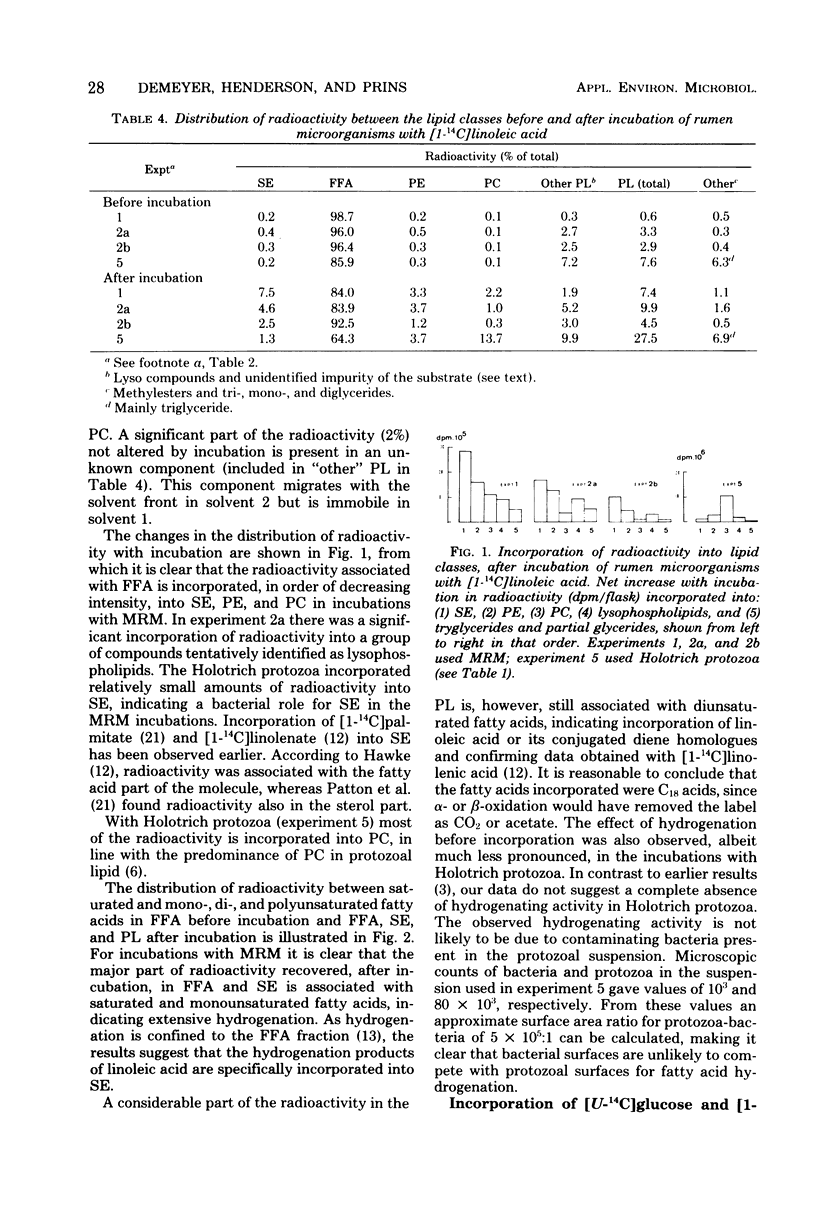
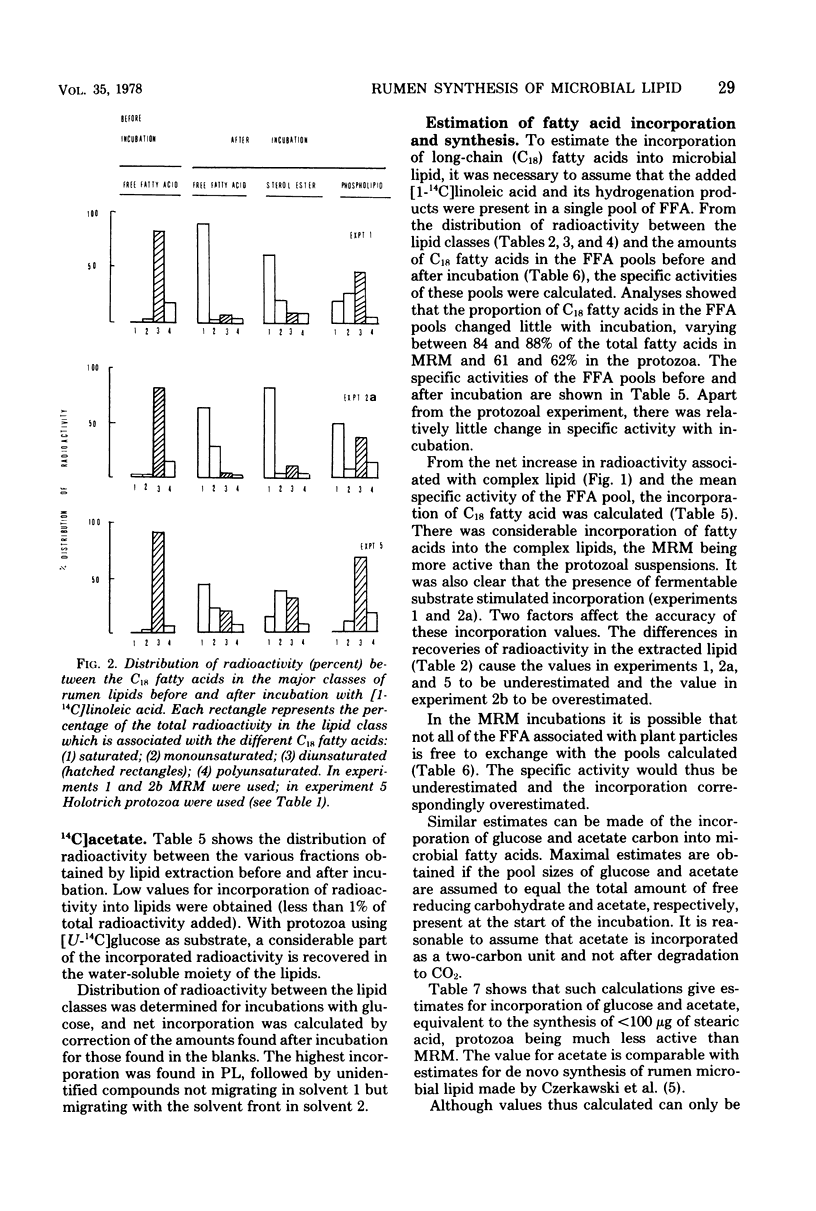
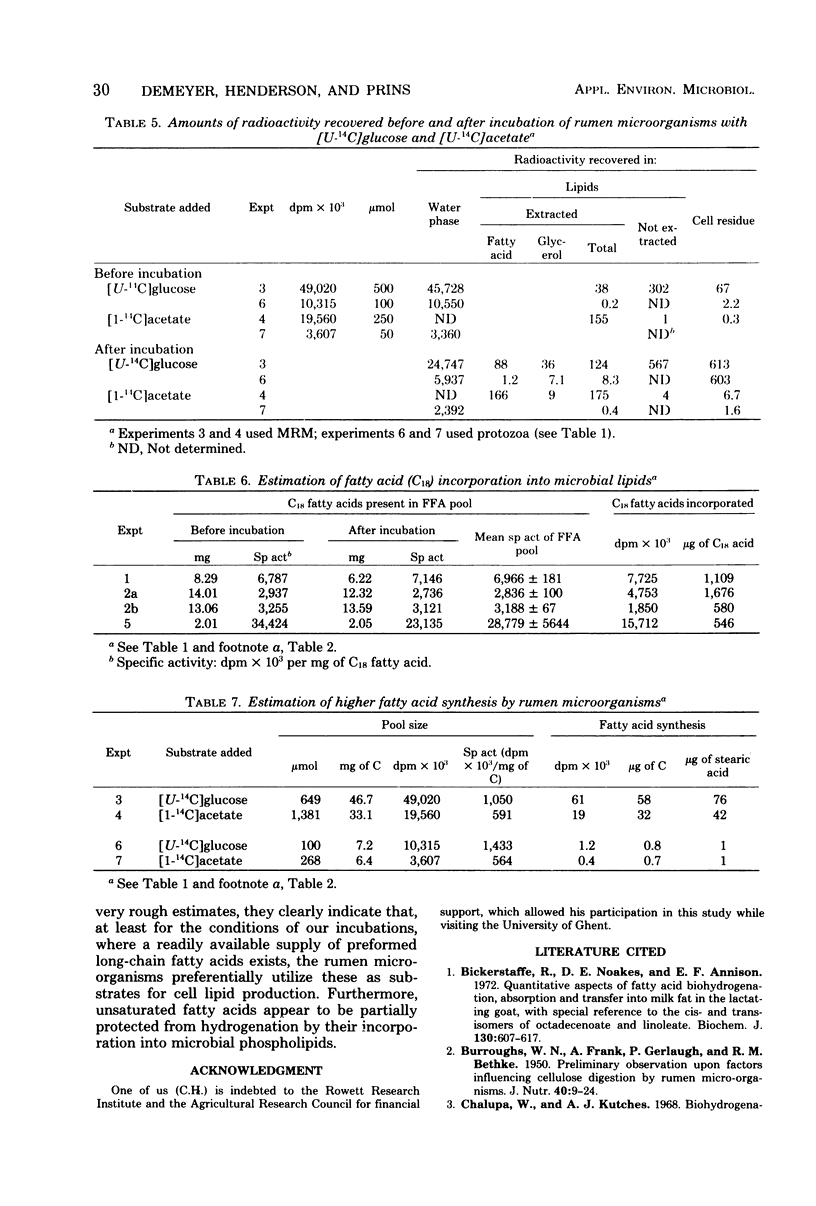
Mixed rumen microorganisms (MRM) or suspensions of rumen Holotrich protozoa obtained from a sheep were incubated anaerobically with [1-14C]linoleic acid, [U-14C]glucose, or [1-14C]acetate. With MRM, the total amount of fatty acids present did not change after incubation. An increase in fatty acids esterified into sterolesters (SE) and polar lipids at the expense of free fatty acids was observed. This effect was intensified by the addition of fermentable carbohydrate to the incubations. Radioactivity from [1-14C]linoleic acid was incorporated into SE and polar lipids with both MRM and Holotrich protozoa. With MRM the order of incorporation of radioactivity was as follows: SE > phosphatidylethanolamine > phosphatidylcholine. With Holotrich protozoa, the order of incorporation was phosphatidylcholine > phosphatidylethanolamine > SE. With MRM the radioactivity remaining in the free fatty acids and that incorporated into SE was mainly associated with saturated fatty acids, but a considerable part of the radioactivity in the polar lipids was associated with dienoic fatty acids. This effect of hydrogenation prior to incorporation was also noted with Holotrich protozoa but to a much lesser extent. Small amounts of radioactivity from [U-14C]glucose and [1-14C]acetate were incorporated into rumen microbial lipids. With protozoa incubated with [U-14C]glucose, the major part of incorporated radioactivity was present in the glycerol moiety of the lipids. From the amounts of lipid classes present, their radioactivity, and fatty acid composition, estimates were made of the amounts of higher fatty acids directly incorporated into microbial lipids and the amounts synthesized de novo from glucose or acetate. It is concluded that the amounts directly incorporated may be greater than the amounts synthesized de novo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURROUGHS W., FRANK N. A. Preliminary observations upon factors influencing cellulose digestion by rumen microorganisms. J Nutr. 1950 Jan;40(1):9–24. doi: 10.1093/jn/40.1.9. [DOI] [PubMed] [Google Scholar]
- Bickerstaffe R., Noakes D. E., Annison E. F. Quantitative aspects of fatty acid biohydrogenation, absorption and transfer into milk fat in the lactating goat, with special reference to the cis- and trans-isomers of octadecenoate and linoleate. Biochem J. 1972 Nov;130(2):607–617. doi: 10.1042/bj1300607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkawski J. W., Christie W. W., Breckenridge G., Hunter M. L. Changes in the rumen metabolism of sheep given increasing amounts of linseed oil in their diet. Br J Nutr. 1975 Jul;34(1):25–44. doi: 10.1017/s0007114575000074. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Kemp P. The aminoethylphosphonate-containing lipids of rumen protozoa. Biochem J. 1967 Nov;105(2):837–842. doi: 10.1042/bj1050837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer D. I., Henderickx H. K. The effect of C18 unsaturated fatty acids of methane production in vitro by mixed rumen bacteria. Biochim Biophys Acta. 1967 Jun 6;137(3):484–497. doi: 10.1016/0005-2760(67)90130-0. [DOI] [PubMed] [Google Scholar]
- Emmanuel B., Milligan L. P., Turner B. V. The metabolism of acetate by rumen microorganisms. Can J Microbiol. 1974 Feb;20(2):183–185. doi: 10.1139/m74-028. [DOI] [PubMed] [Google Scholar]
- Emmanuel B. On the origin of rumen protozoan fatty acids. Biochim Biophys Acta. 1974 Mar 28;337(3):404–413. doi: 10.1016/0005-2760(74)90116-7. [DOI] [PubMed] [Google Scholar]
- Harfoot C. G., Crouchman M. L., Noble R. C., Moore J. H. Competition between food particles and rumen bacteria in the uptake of long-chain fatty acids and triglycerides. J Appl Bacteriol. 1974 Dec;37(4):633–641. doi: 10.1111/j.1365-2672.1974.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Hawke J. C., Silcock W. R. Lipolysis and hydrogenation in the rumen. Biochem J. 1969 Mar;112(1):131–132. doi: 10.1042/bj1120131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke J. C. The incorporation of long-chain fatty acids into lipids by rumen bacteria and the effect on biohydrogenation. Biochim Biophys Acta. 1971 Nov 5;248(2):167–170. doi: 10.1016/0005-2760(71)90003-8. [DOI] [PubMed] [Google Scholar]
- Hungate R. E. Studies on Cellulose Fermentation: III. The Culture and Isolation for Cellulose-decomposing Bacteria from the Rumen of Cattle. J Bacteriol. 1947 May;53(5):631–645. doi: 10.1128/jb.53.5.631-645.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp A. J., Blanchard F. A. Quench correction in Cerenkov counting: channels ration and external source channels ratio methods. Anal Biochem. 1971 Dec;44(2):369–380. doi: 10.1016/0003-2697(71)90222-3. [DOI] [PubMed] [Google Scholar]
- Kates M. Biosynthesis of lipids in microorganisms. Annu Rev Microbiol. 1966;20:13–44. doi: 10.1146/annurev.mi.20.100166.000305. [DOI] [PubMed] [Google Scholar]
- Katz I., Keeney M. Characterization of the octadecenoic acids in rumen digesta and rumen bacteria. J Dairy Sci. 1966 Aug;49(8):962–966. doi: 10.3168/jds.S0022-0302(66)87990-0. [DOI] [PubMed] [Google Scholar]
- Katz I., Keeney M. The lipids of some rumen holotrich protozoa. Biochim Biophys Acta. 1967 Aug 8;144(1):102–112. doi: 10.1016/0005-2760(67)90081-1. [DOI] [PubMed] [Google Scholar]
- Outen G. E., Beever D. E., Osbourn D. F. Digestion and absorption of lipids by sheep fed chopped and ground dried grass. J Sci Food Agric. 1974 Aug;25(8):981–987. doi: 10.1002/jsfa.2740250814. [DOI] [PubMed] [Google Scholar]
- PRIVETT O. S., BLANK M. L., ROMANUS O. ISOLATION ANALYSIS OF TISSUE FATTY ACIDS BY ULTRAMICRO-OZONOLYSIS IN CONJUNCTION WITH THIN-LAYER CHROMATOGRAPHY AND GAS-LIQUID CHROMATOGRAPHY. J Lipid Res. 1963 Jul;4:260–265. [PubMed] [Google Scholar]
- Patton R. A., McCarthy R. D., Griel L. C., Jr Lipid synthesis by rumen microorganisms. II. Further characterization of the effects of methionine. J Dairy Sci. 1970 Apr;53(4):460–465. doi: 10.3168/jds.s0022-0302(70)86231-2. [DOI] [PubMed] [Google Scholar]
- Prins R. A., Prast E. R. Oxidation of NADH in a coupled oxidase-peroxidase reaction and its significance for the fermentation in rumen protozoa of the genus Isotricha. J Protozool. 1973 Aug;20(3):471–477. doi: 10.1111/j.1550-7408.1973.tb00929.x. [DOI] [PubMed] [Google Scholar]
- SNYDER F. RADIOASSAY OF THIN-LAYER CHROMATOGRAMS: A HIGH-RESOLUTION ZONAL SCRAPER FOR QUANTITATIVE C14 AND H3 SCANNING OF THIN-LAYER CHROMATOGRAMS. Anal Biochem. 1964 Oct;9:183–196. doi: 10.1016/0003-2697(64)90102-2. [DOI] [PubMed] [Google Scholar]
- Sklan D., Budowski P., Volcani R. Synthesis in vitro of linoleic acid by rumen liquor of calves. Br J Nutr. 1972 Sep;28(2):239–248. doi: 10.1079/bjn19720030. [DOI] [PubMed] [Google Scholar]
- Sklan D., Volcani R., Budowski P. Formation of octadecadienoic acid by rumen liquor of calves, cows and sheep in vitro. J Dairy Sci. 1971 Apr;54(4):515–519. doi: 10.3168/jds.S0022-0302(71)85877-0. [DOI] [PubMed] [Google Scholar]
- Tichý J., Dencker S. J. Separation of cholesterol esters. A comparison between paper and thin layer chromatography. J Chromatogr. 1968 Mar 12;33(2):262–266. doi: 10.1016/s0021-9673(00)98646-1. [DOI] [PubMed] [Google Scholar]
- VIVIANI R., LENAZ G. SINTESI DI ACIDI GRASSI A LUNGA CATENA NEL RUMINE DI OVINO. Boll Soc Ital Biol Sper. 1963 Dec 31;39:1836–1839. [PubMed] [Google Scholar]
- Viviani R. Metabolism of long-chain fatty acids in the rumen. Adv Lipid Res. 1970;8:267–346. doi: 10.1016/b978-0-12-024908-4.50013-1. [DOI] [PubMed] [Google Scholar]
- WILLIAMS P. P., GUTIERREZ J., DAVIS R. E. Lipid metabolism of rumen ciliates and bacteria. II. Uptake of fatty acids and lipid analysis of Isotricha intestinalis and rumen bacteria with further information on Entodinium simplex. Appl Microbiol. 1963 May;11:260–264. doi: 10.1128/am.11.3.260-264.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


