Abstract
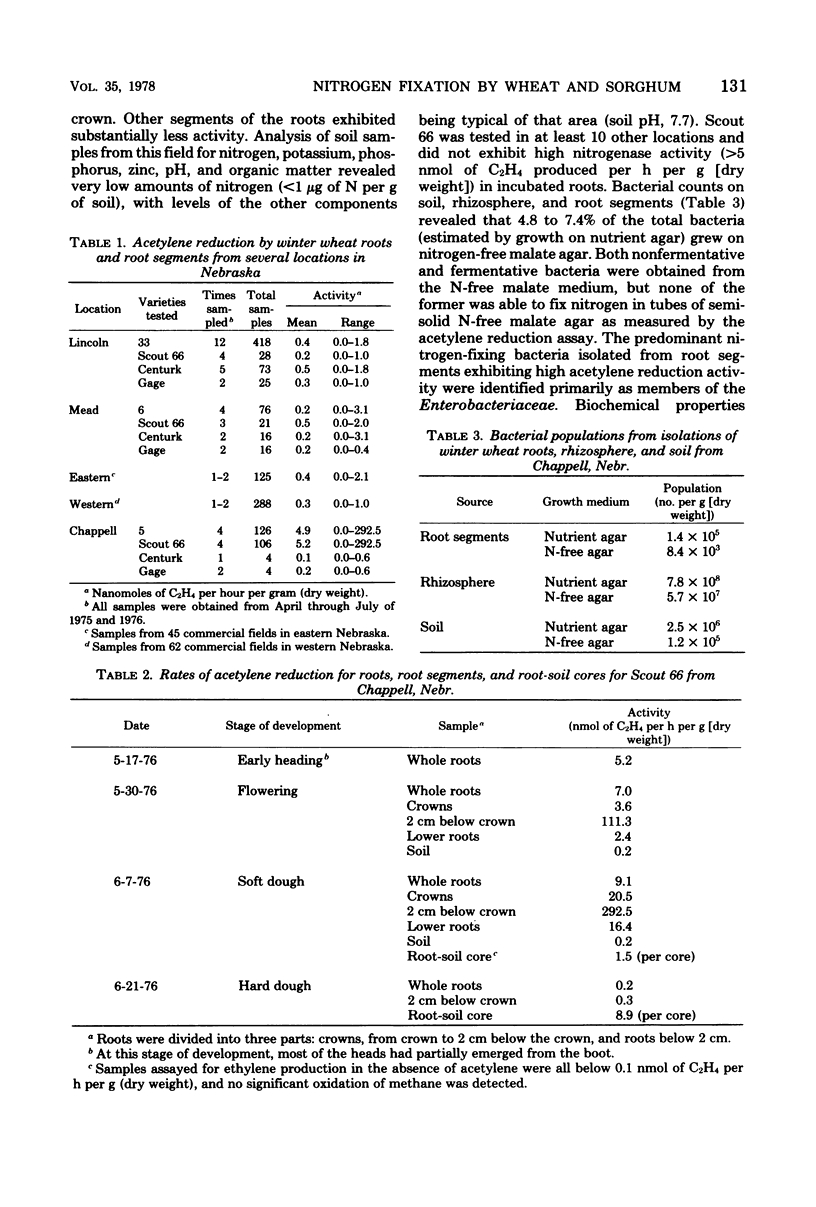
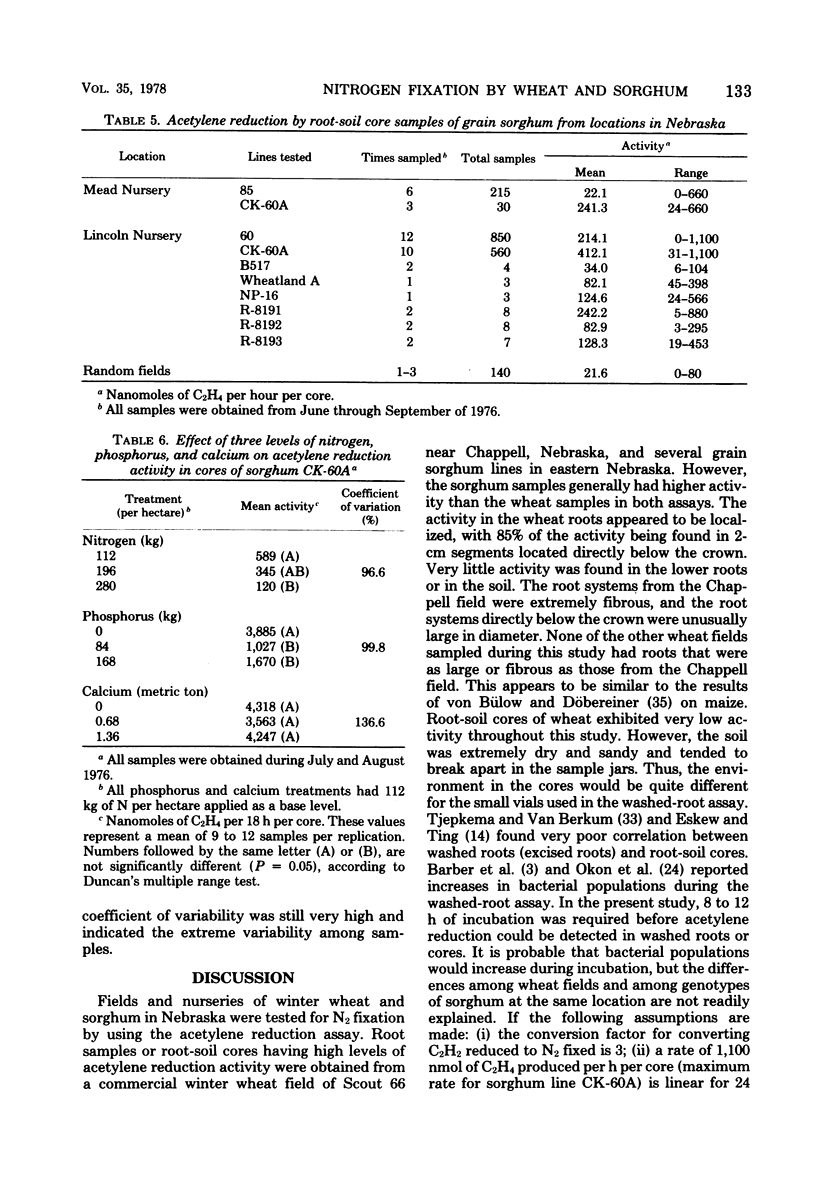
Root segments and root-soil cores (6.5-cm diameter) from fields and nurseries of winter wheat and sorghum were tested for N2 fixation by using the acetylene reduction assay. Wheat samples (approximately 1,200) from 109 sites generally had low or no activity (0 to 3.1 nmol of C2H4 produced per h per g [dry weight] of root segments), even after 24 h of incubation. However, a commercial field of Scout 66, located in western Nebraska, exhibited appreciable activity (290 nmol of C2H4 produced per h per g [dry weight] of root segments). Of 400 sorghum lines and crosses, grain sorghums (i.e., CK-60A, Wheatland A, B517, and NP-16) generally exhibited higher nitrogenase activity than forage sorghums or winter wheats. CK-60A, a male sterile grain sorghum, was sampled at four locations and had the most consistent activity of 24 to 1,100 nmol of C2H4 produced per h per core. The maximum rate extrapolated to 2.5 g of N per hectare per day. Numerous N2-fixing bacterial isolates were obtained from wheat and sorghum roots that exhibited high nitrogenase activity. Most isolates were members of the Enterobacteriacae, i.e., Klebsiella pneumoniae, Enterobacter cloacae, and Erwinia herbicola.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber L. E., Tjepkema J. D., Russell S. A., Evans H. J. Acetylene reduction (nitrogen fixation) associated with corn inoculated with Spirillum. Appl Environ Microbiol. 1976 Jul;32(1):108–113. doi: 10.1128/aem.32.1.108-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Campbell N. E., Hill S. Asymbiotic nitrogen-fixing bacteria from the surfaces of nodules and roots of legumes. Can J Microbiol. 1972 Jan;18(1):13–21. doi: 10.1139/m72-003. [DOI] [PubMed] [Google Scholar]
- Neilson A. H., Sparell L. Acetylene reduction (nitrogen fixation) by enterobacteriaceae isolated from paper mill process waters. Appl Environ Microbiol. 1976 Aug;32(2):197–205. doi: 10.1128/aem.32.2.197-205.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. D., Barber L. E., Tjepkema J., Russell S. A., Powelson R., Evans H. J. Nitrogen fixation associated with grasses in Oregon. Can J Microbiol. 1976 Apr;22(4):523–530. doi: 10.1139/m76-078. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Methods for Growing Spirillum lipoferum and for Counting It in Pure Culture and in Association with Plants. Appl Environ Microbiol. 1977 Jan;33(1):85–88. doi: 10.1128/aem.33.1.85-88.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W. A., Paul E. A. The acetylene reduction assay for measuring nitrogen fixation in waterlogged soil. Can J Microbiol. 1971 Aug;17(8):1049–1056. doi: 10.1139/m71-166. [DOI] [PubMed] [Google Scholar]
- Tjepkema J., Van Berkum P. Acetylene reduction by soil cores of maize and sorghum in Brazil. Appl Environ Microbiol. 1977 Mar;33(3):626–629. doi: 10.1128/aem.33.3.626-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bülow J. F., Döbereiner J. Potential for nitrogen fixation in maize genotypes in Brazil. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2389–2393. doi: 10.1073/pnas.72.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]


