Abstract
This paper describes the international collaborative assay that led to the establishment in 1967 of the International Reference Preparation of Influenza Virus Haemagglutinin (Type A) and the studies completed during the following years on the use of the preparation for evaluating the haemagglutinin content of 46 influenza virus vaccines in terms of international units. The WHO Expert Committee on Biological Standardization (1967) defined the International Unit as 0,09361 mg of the International Reference Preparation.
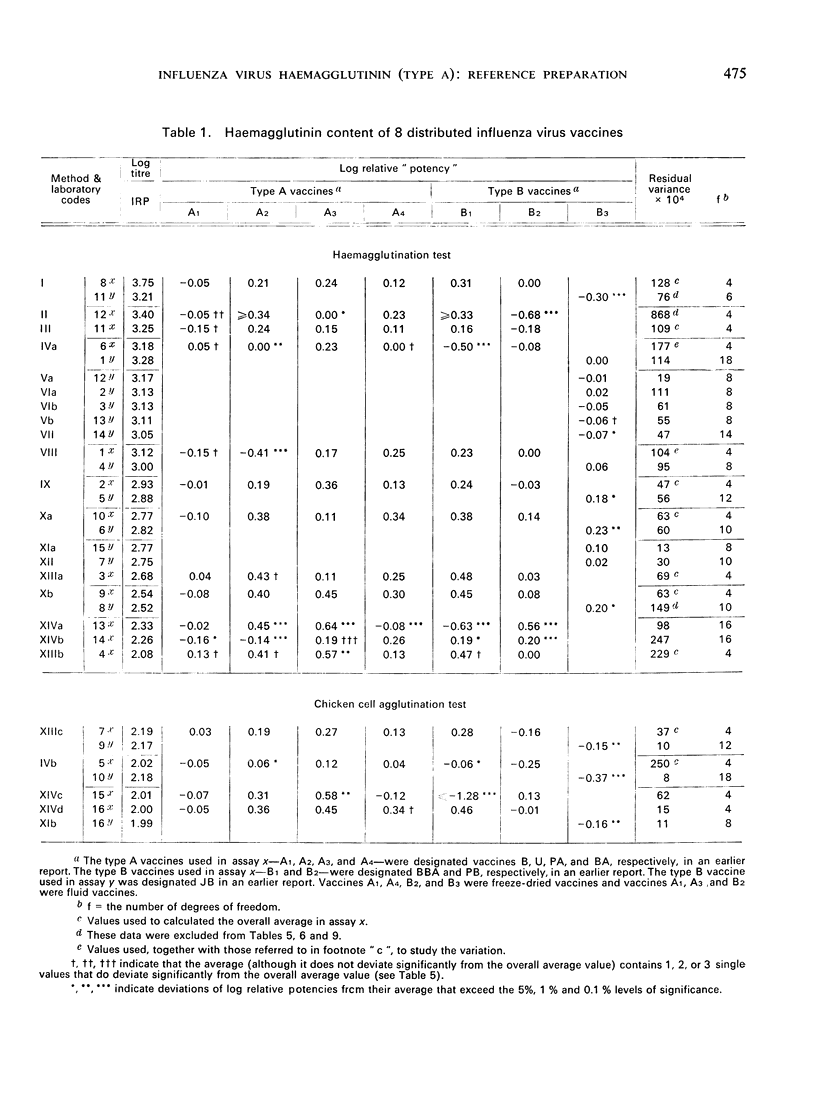
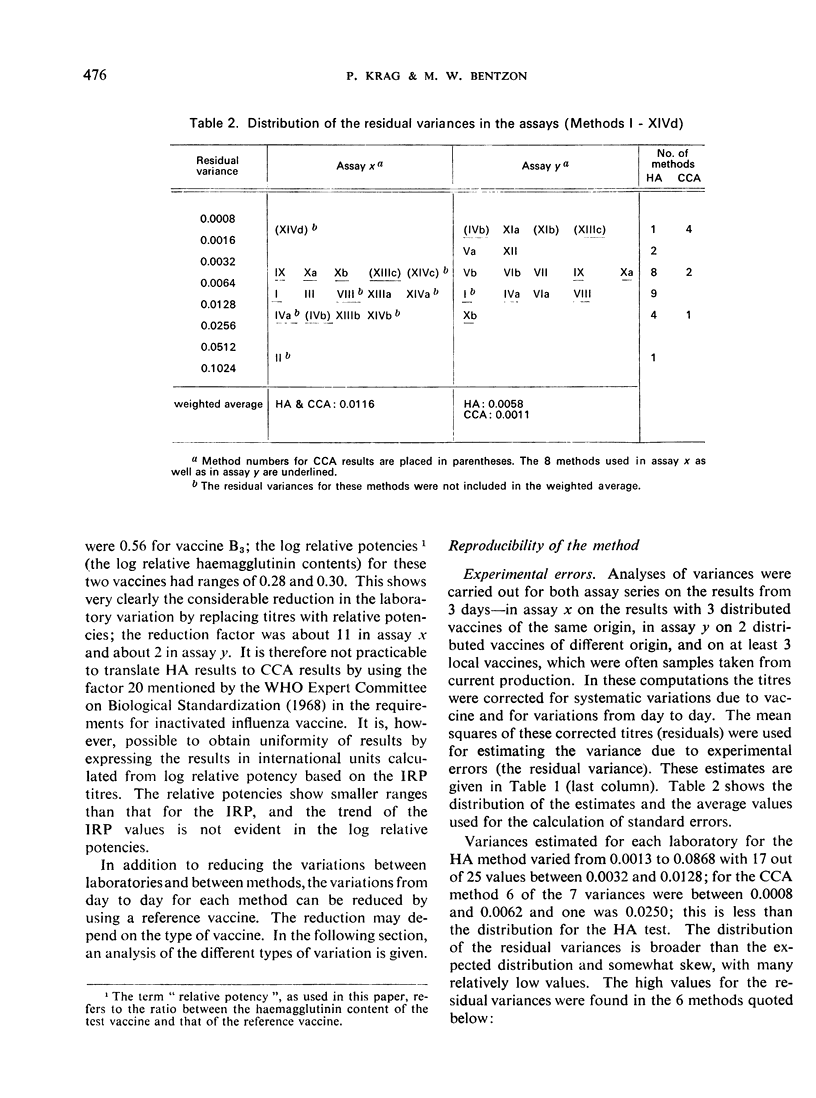
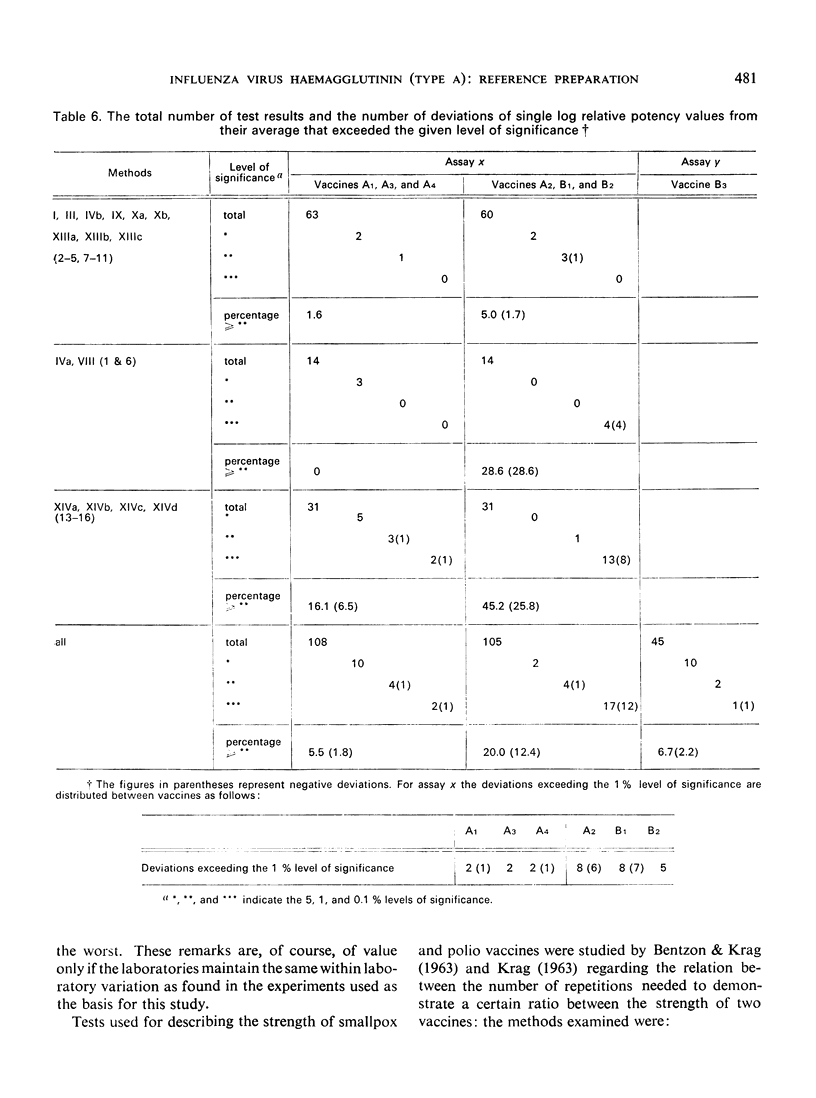
Altogether 14 laboratories in 12 countries took part in one or both studies, using a total of 24 methods (HA titrations and, in a few cases CCA titrations). Major differences in the HA titres were found between laboratories, while the potencies (the haemagglutinin content values) relative to the International Reference Preparation were free from most of these differences. Haemagglutination titres varied over a range factor up to 50, while the corresponding relative ”potencies” varied with a factor of only 2. The CCA method used in a few laboratories gave results close to the lowest haemagglutination titres and showed relatively small variations between laboratories. The analyses of variance disclosed differences in the variation within laboratories, but for the majority of the laboratories the variation allowed an overall estimate of a standard error.
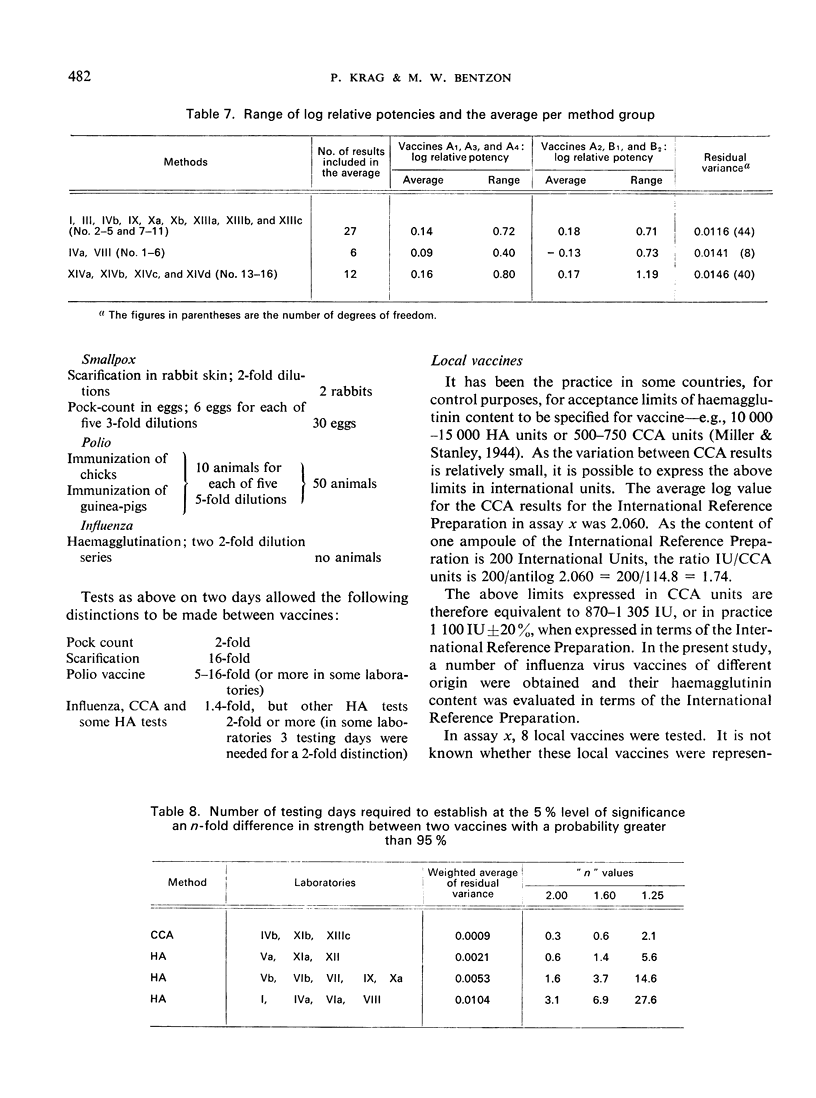
The calculation of haemagglutinin content (in IU) from relative potencies is described. Advice is given on the selection, preparation, and titration of a local reference vaccine with a view to expressing its haemagglutinin content in international units.
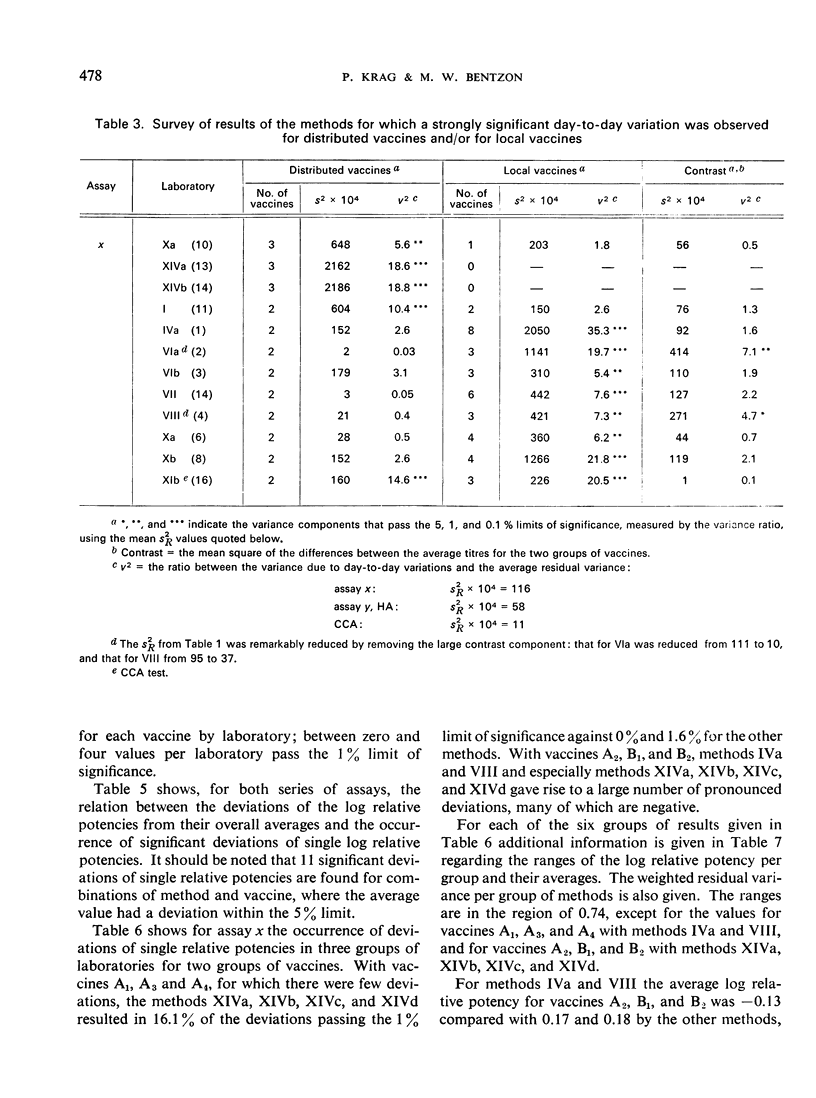
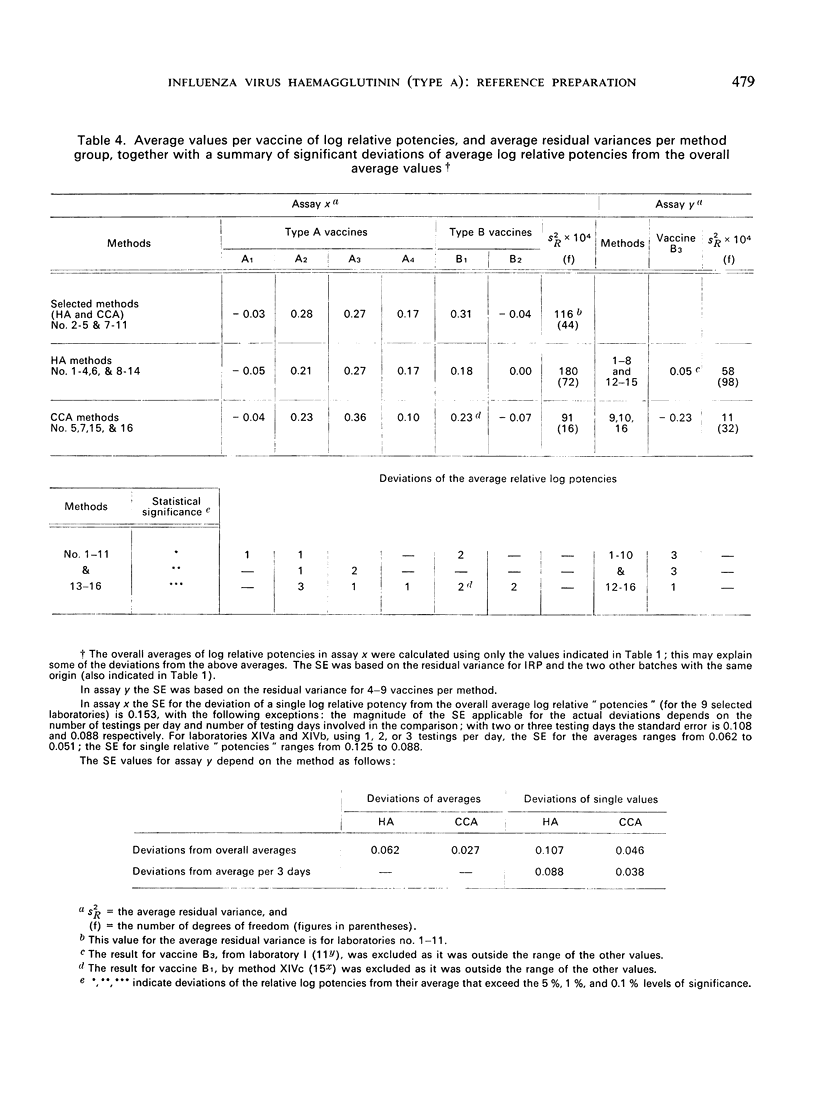
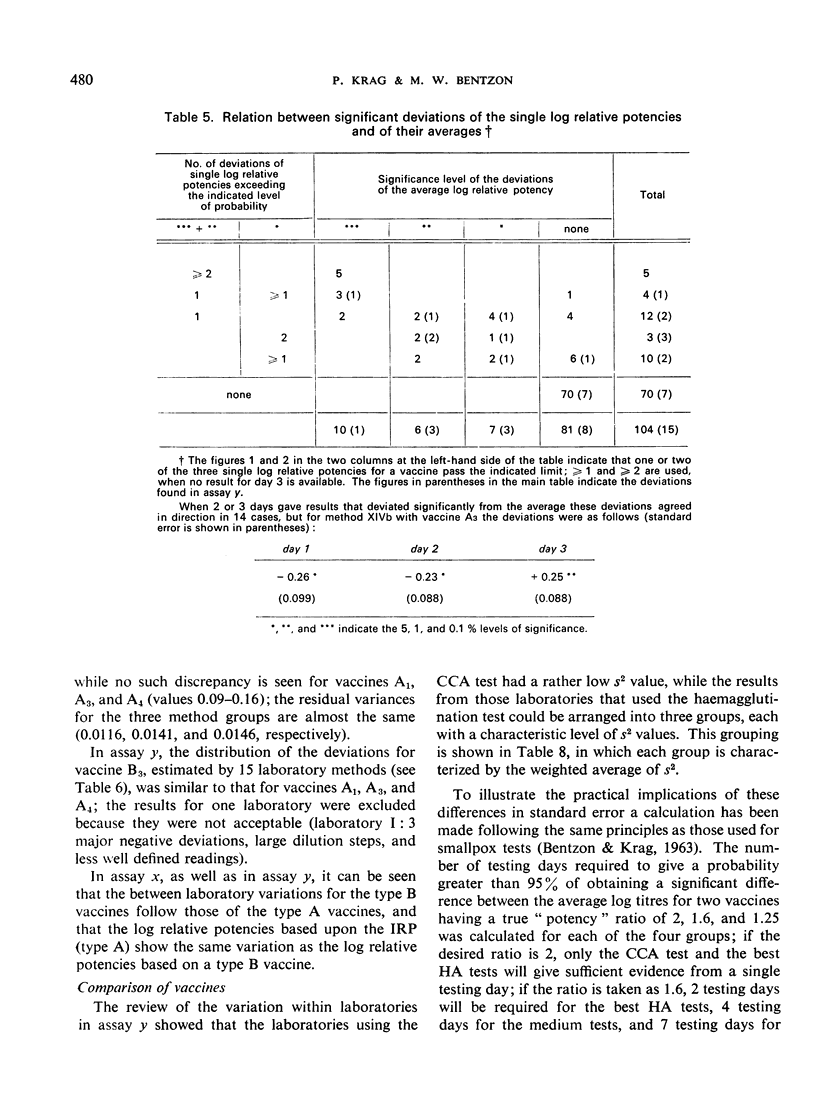
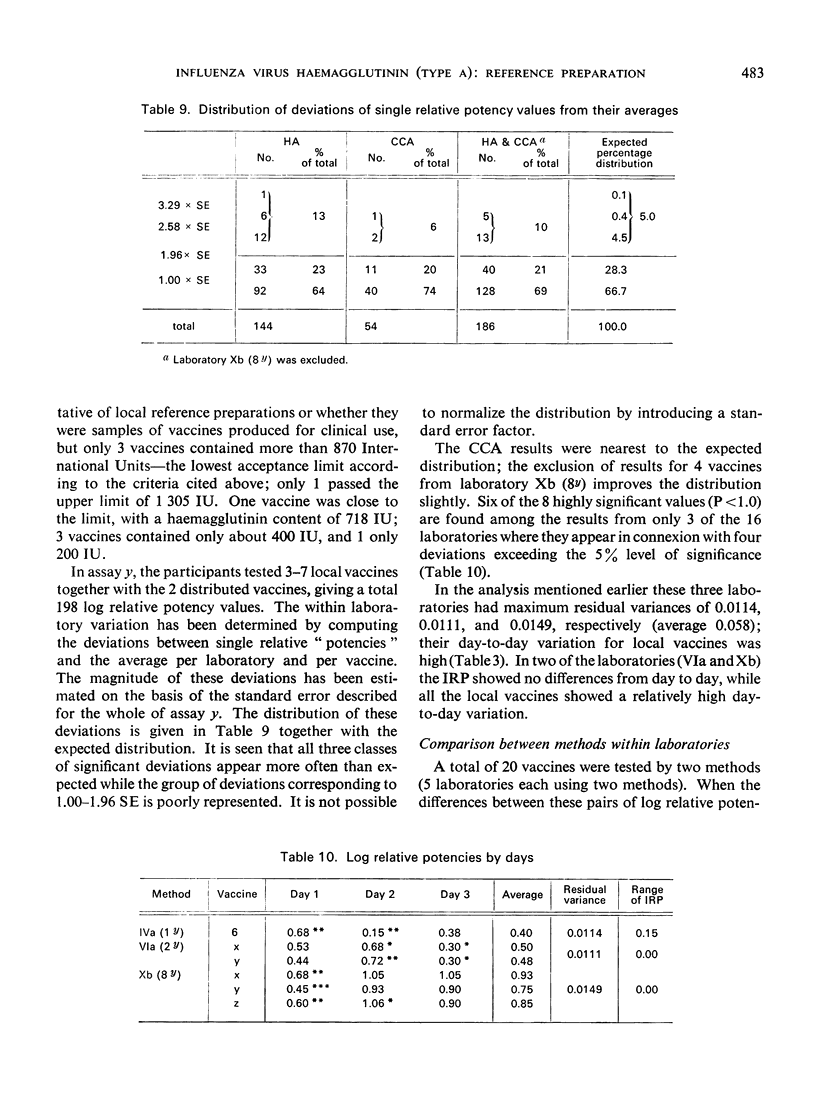
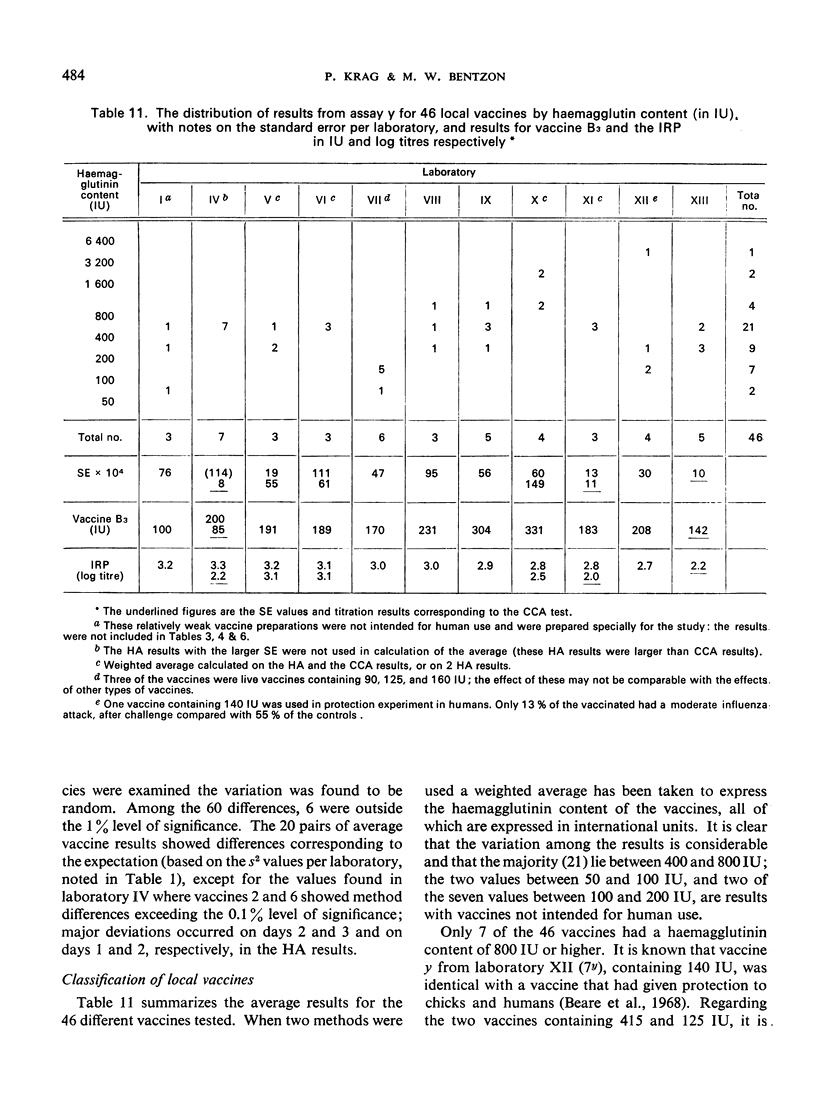
The test results with 46 local vaccines are also given. The deviations of the relative potencies from the average per vaccine showed a distribution with eight major discrepancies instead of the expected one. The background for these cases is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENTZON M. W., KRAG P. EVALUATION OF RESULTS OF THE TESTING OF SMALLPOX VACCINES. A REPORT ON STATISTICAL METHODS. Bull World Health Organ. 1963;29:745–751. [PMC free article] [PubMed] [Google Scholar]
- Beare A. S., Hobson D., Reed S. E., Tyrrell D. A. A comparison of live and killed influenza-virus vaccines. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet. 1968 Aug 24;2(7565):418–422. doi: 10.1016/s0140-6736(68)90463-7. [DOI] [PubMed] [Google Scholar]
- Miller G. L., Stanley W. M. QUANTITATIVE ASPECTS OF THE RED BLOOD CELL AGGLUTINATION TEST FOR INFLUENZA VIRUS. J Exp Med. 1944 Feb 1;79(2):185–195. doi: 10.1084/jem.79.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]


