Abstract
The results of research on the immunogenicity of experimental mycobacterial vaccines are characterized by a remarkable lack of agreement about which substances are most immunogenic. The disagreement has usually been attributed to the differences in the methods of preparing the vaccines. An alternative hypothesis is that the conflicting results are a product of the different methods used to assess the potency of the vaccines.
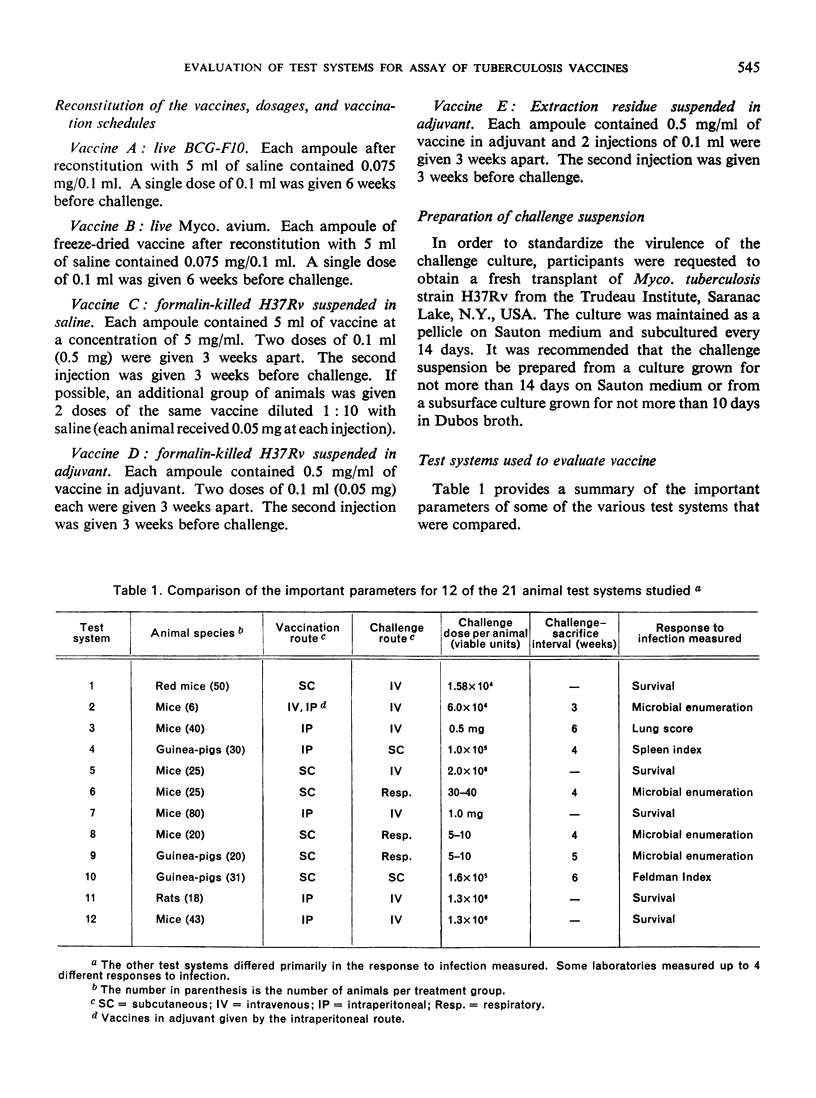
To determine if the method by which a vaccine is tested is a major factor contributing to the disagreement, an experiment was conducted in which a series of five different vaccines was distributed to each of nine participating laboratories. Each investigator evaluated the potency of the vaccines in one or more animal models of his own choosing. This in effect held the method of vaccine preparation constant while permitting all other variables to change.
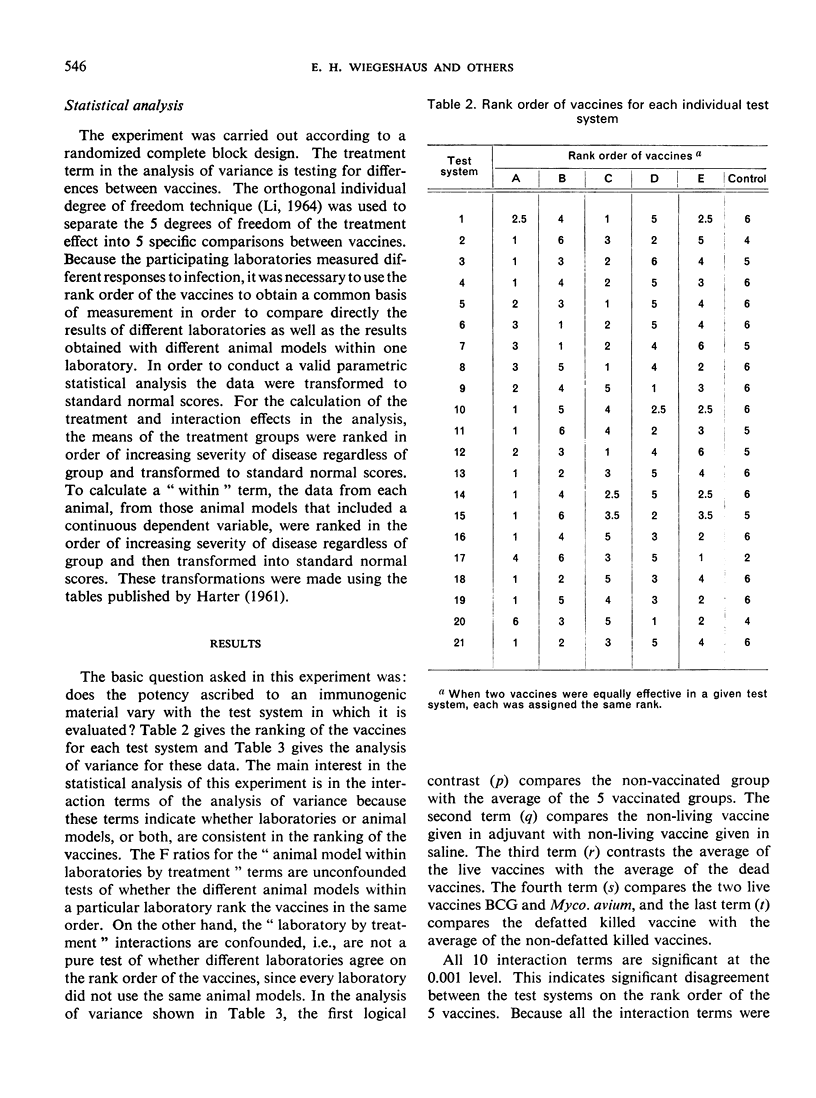
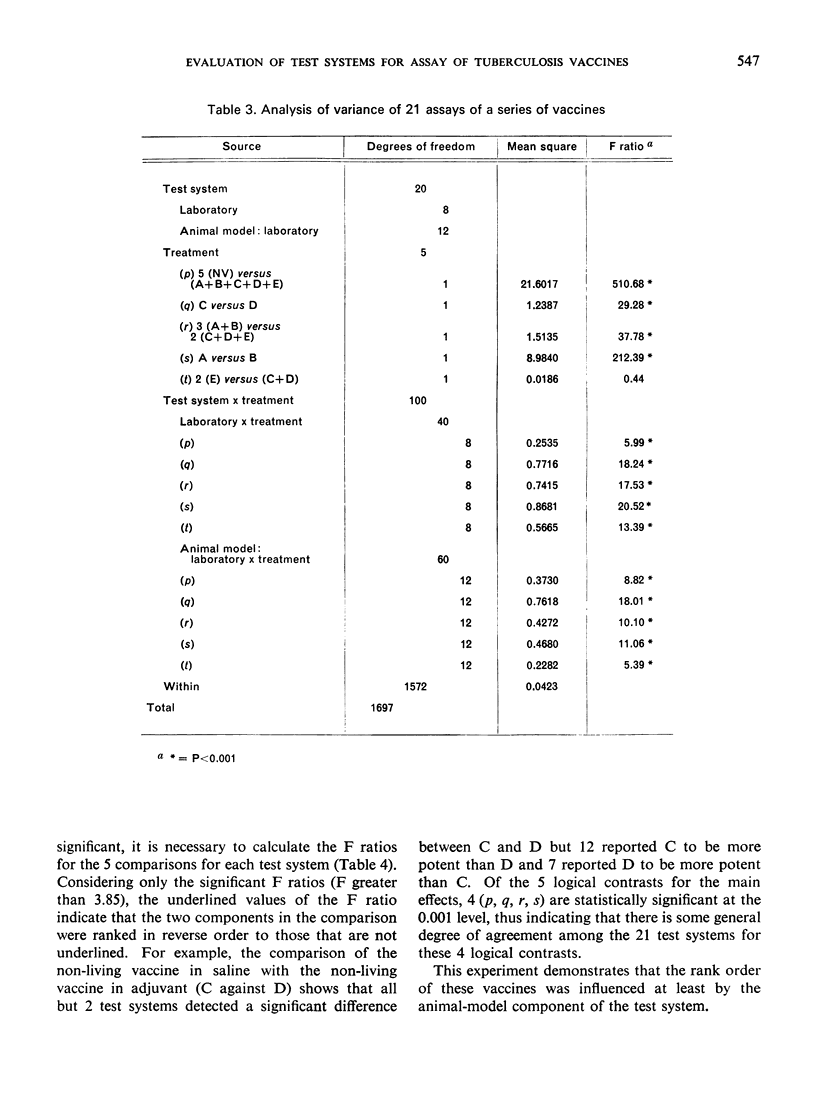
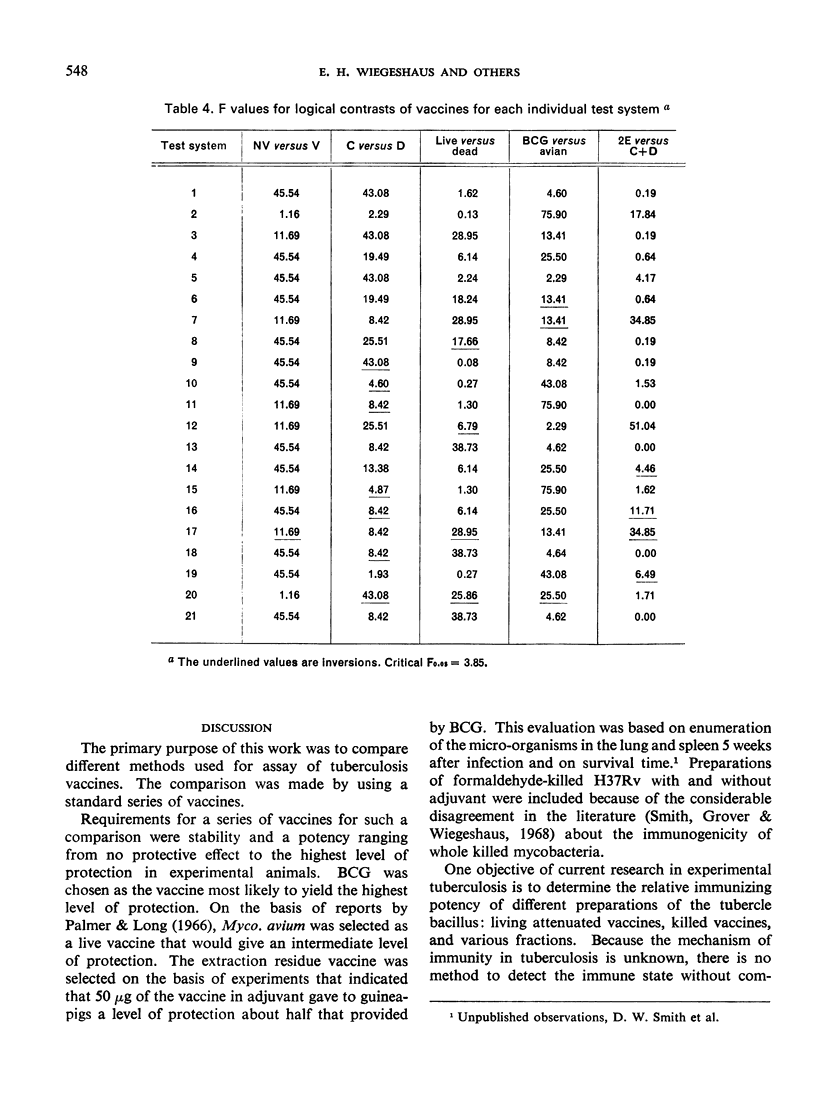
The ranking of the five vaccines was random, thus demonstrating that the method by which a vaccine is tested influences the apparent potency of a vaccine. These results cast doubt on the conclusions about the relative potency of tuberculosis vaccines evaluated by different methods.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FREGNAN G. B., SMITH D. W. Immunogenicity and allergenioity in guinea pigs of a defatted mycobacterial vaccine and its fractions. Am Rev Respir Dis. 1963 Jun;87:877–888. doi: 10.1164/arrd.1963.87.6.877. [DOI] [PubMed] [Google Scholar]
- Palmer C. E., Long M. W. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am Rev Respir Dis. 1966 Oct;94(4):553–568. doi: 10.1164/arrd.1966.94.4.553. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Grover A. A., Wiegeshaus E. Nonliving immunogenic substances of Mycobacteria. Bibl Tuberc. 1968;24:191–227. [PubMed] [Google Scholar]
- UNGAR J., MUGGLETON P. W., DUDLEY J. A., GRIFFITHS M. I. Preparation and properties of a freeze-dried B.C.G. vaccine of increased stability. Br Med J. 1962 Oct 27;2(5312):1086–1089. doi: 10.1136/bmj.2.5312.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]


