Abstract
The general types of biological reaction that are most prominent in the modification of organophosphorus compounds involve the mixed-function oxidases, hydrolases, or transferases. In certain cases, more than one of these reactions may be involved at the same site on the pesticide molecule. Examples of various organophosphorus pesticides that are altered by oxidation, hydrolysis, alkyl- or aryl-group transfer, reduction, and conjugation are discussed. The increase or decrease in toxicity of a pesticide that can result from biological modification is emphasized.
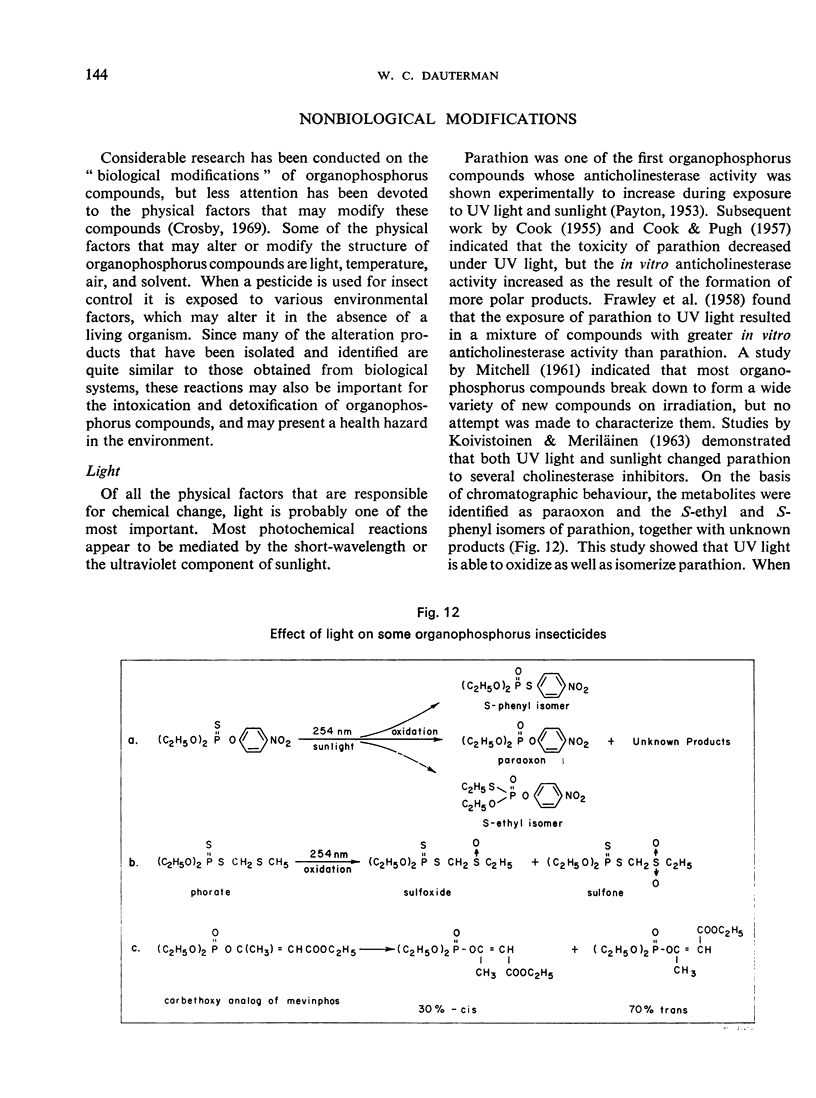
Non-biological transformations of organophosphorus compounds involve the effect ou the compounds of such factors as light, air, temperature, and solvent. These factors are discussed with special emphasis on desulfuration, rearrangement, and oxidation.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
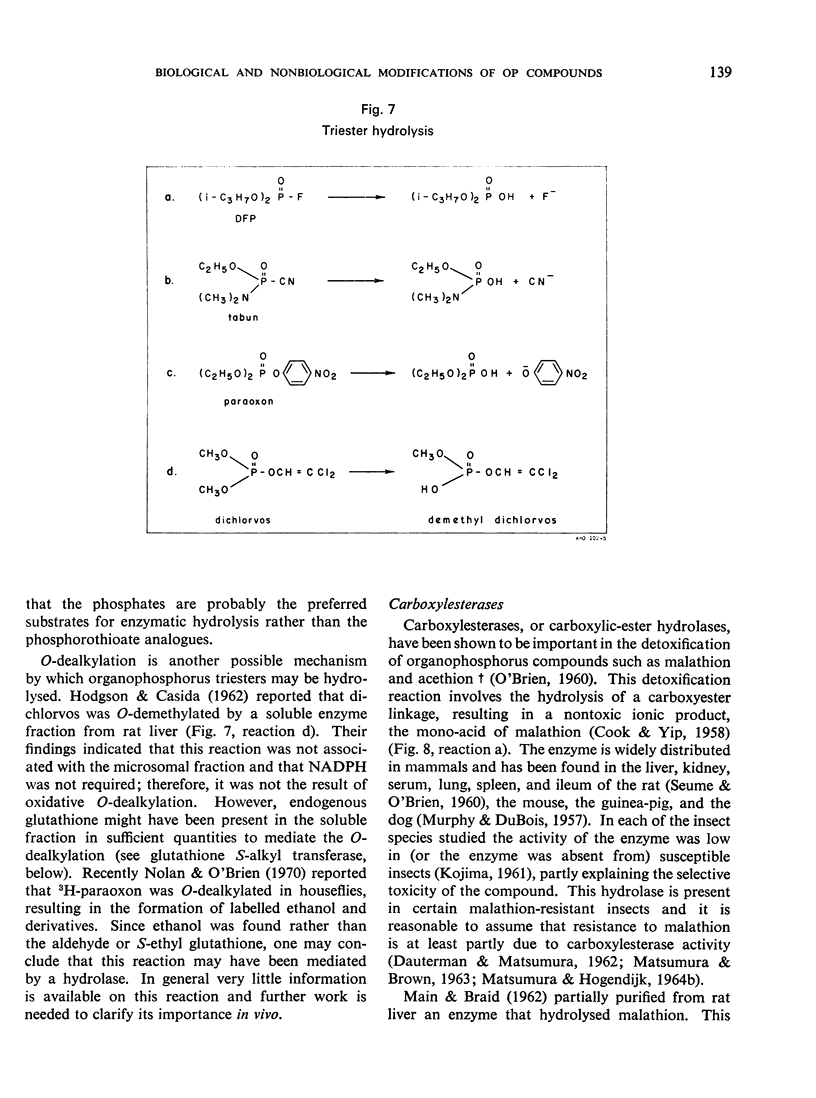
- ADIE P. A. The purification of sarinase from bovine plasma. Can J Biochem Physiol. 1956 Nov;34(6):1091–1094. [PubMed] [Google Scholar]
- ALDRIDGE W. N. Serum esterases. II. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem J. 1953 Jan;53(1):117–124. doi: 10.1042/bj0530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUGUSTINSSON K. B., JONSSON G. Biochemical studies on the degradation products of diazinone. Experientia. 1957 Nov 15;13(11):438–440. doi: 10.1007/BF02157237. [DOI] [PubMed] [Google Scholar]
- Andrawes N. R., Dorough H. W., Lindquist D. A. Degradation and elimination of temik in rats. J Econ Entomol. 1967 Aug;60(4):979–987. doi: 10.1093/jee/60.4.979. [DOI] [PubMed] [Google Scholar]
- BULL D. L. METABOLISM OF DI-SYSTON BY INSECTS, ISOLATED COTTON LEAVES, AND RATS. J Econ Entomol. 1965 Apr;58:249–254. doi: 10.1093/jee/58.2.249. [DOI] [PubMed] [Google Scholar]
- CASIDA J. E. Isomeric substituted-vinyl phosphates as systemic insecticides. Science. 1955 Sep 30;122(3170):597–598. doi: 10.1126/science.122.3170.597-a. [DOI] [PubMed] [Google Scholar]
- COHEN J. A., WARRINGA M. G. Purification and properties of dialkylfluorophosphatase. Biochim Biophys Acta. 1957 Oct;26(1):29–39. doi: 10.1016/0006-3002(57)90050-1. [DOI] [PubMed] [Google Scholar]
- Colinese D. L., Terry H. J. Phosalone--a wide spectrum organo-phosphorus insecticide. Chem Ind. 1968 Nov 2;44:1507–1511. [PubMed] [Google Scholar]
- Crosby D. G. 3. Metabolism of pesticides. The nonmetabolic decomposition of pesticides. Ann N Y Acad Sci. 1969;160(1):82–96. doi: 10.1111/j.1749-6632.1969.tb15829.x. [DOI] [PubMed] [Google Scholar]
- DAUTERMAN W. C., MATSUMURA F. Effect of malathion analogs upon resistant and susceptible Culex trasalis mosquitoes. Science. 1962 Nov 9;138(3541):694–695. doi: 10.1126/science.138.3541.694. [DOI] [PubMed] [Google Scholar]
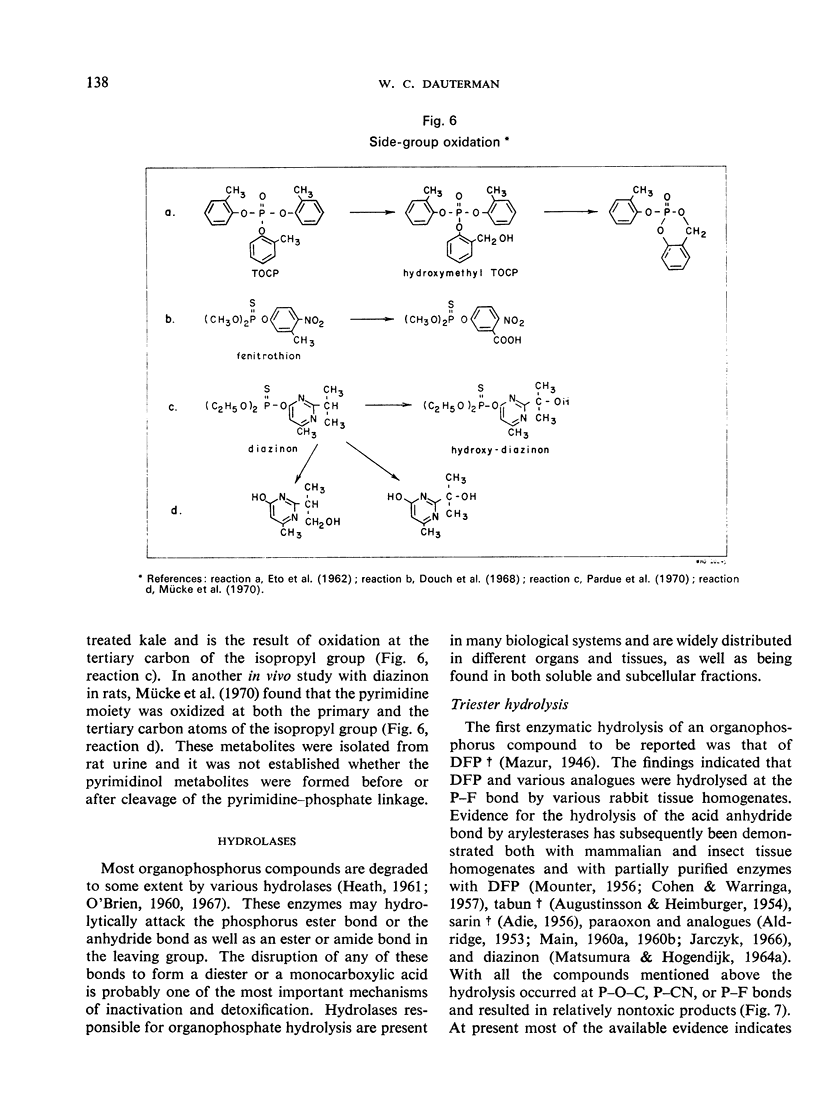
- ETO M., CASIDA J. E., ETO T. Hydroxylation and cyclization reactions involved in the metabolism of tri-o-cresyl phosphate. Biochem Pharmacol. 1962 Apr-May;11:337–352. doi: 10.1016/0006-2952(62)90056-4. [DOI] [PubMed] [Google Scholar]
- Eto M., Oshima Y., Casida J. E. Plasma albumin as a catalyst in cyclization of diaryl o-(alpha-hydroxy)tolyl phosphates. Biochem Pharmacol. 1967 Feb;16(2):295–308. doi: 10.1016/0006-2952(67)90031-7. [DOI] [PubMed] [Google Scholar]
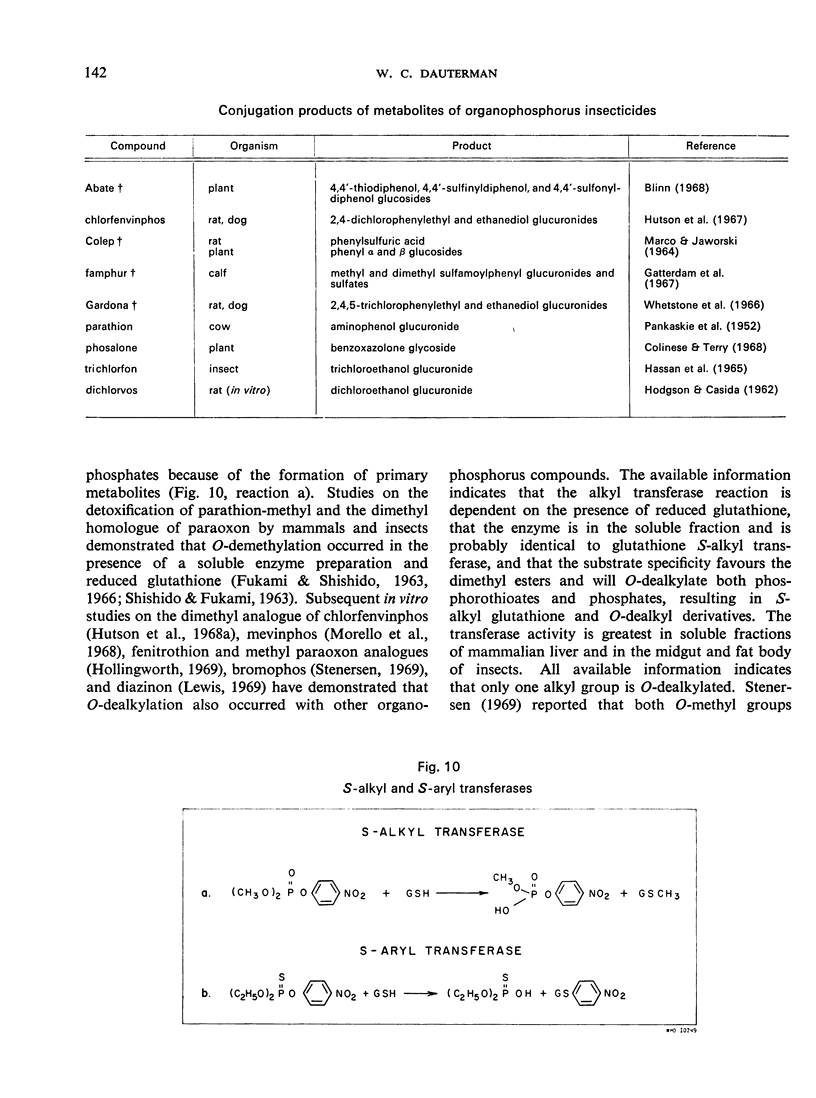
- Fukami J. I., Shishido T. Nature of a soluble, glutathione-dependent enzyme system active in cleavage of methyl parathion to desmethyl parathion. J Econ Entomol. 1966 Dec;59(6):1338–1346. doi: 10.1093/jee/59.6.1338. [DOI] [PubMed] [Google Scholar]
- Fukuto T. R., Metcalf R. L. Metabolism of insecticides in plants and animals. Ann N Y Acad Sci. 1969;160(1):97–113. doi: 10.1111/j.1749-6632.1969.tb15830.x. [DOI] [PubMed] [Google Scholar]
- GAGE J. C. A cholinesterase inhibitor derived from OO-diethyl O-p-nitrophenyl thiophosphate in vivo. Biochem J. 1953 Jun;54(3):426–430. doi: 10.1042/bj0540426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A., Zayed S. M., Abdel-Hamid F. M. Metabolism of organophosphorus insecticides. V. Mechanism of detoxification of Dipterex in Prodenia litura F. Biochem Pharmacol. 1965 Nov;14(11):1577–1584. doi: 10.1016/0006-2952(65)90012-2. [DOI] [PubMed] [Google Scholar]
- Hitchcock M., Murphy S. D. Enzymatic reduction of O,O-(4-nitrophenyl) phosphorothioate, O,O-diethyl O-(4-nitrophenyl) phosphate, and O-ethyl O-(4-nitrophenyl) benzene thiophosphonate by tissues from mammals, birds and fishes. Biochem Pharmacol. 1967 Sep 9;16(9):1801–1811. doi: 10.1016/0006-2952(67)90257-2. [DOI] [PubMed] [Google Scholar]
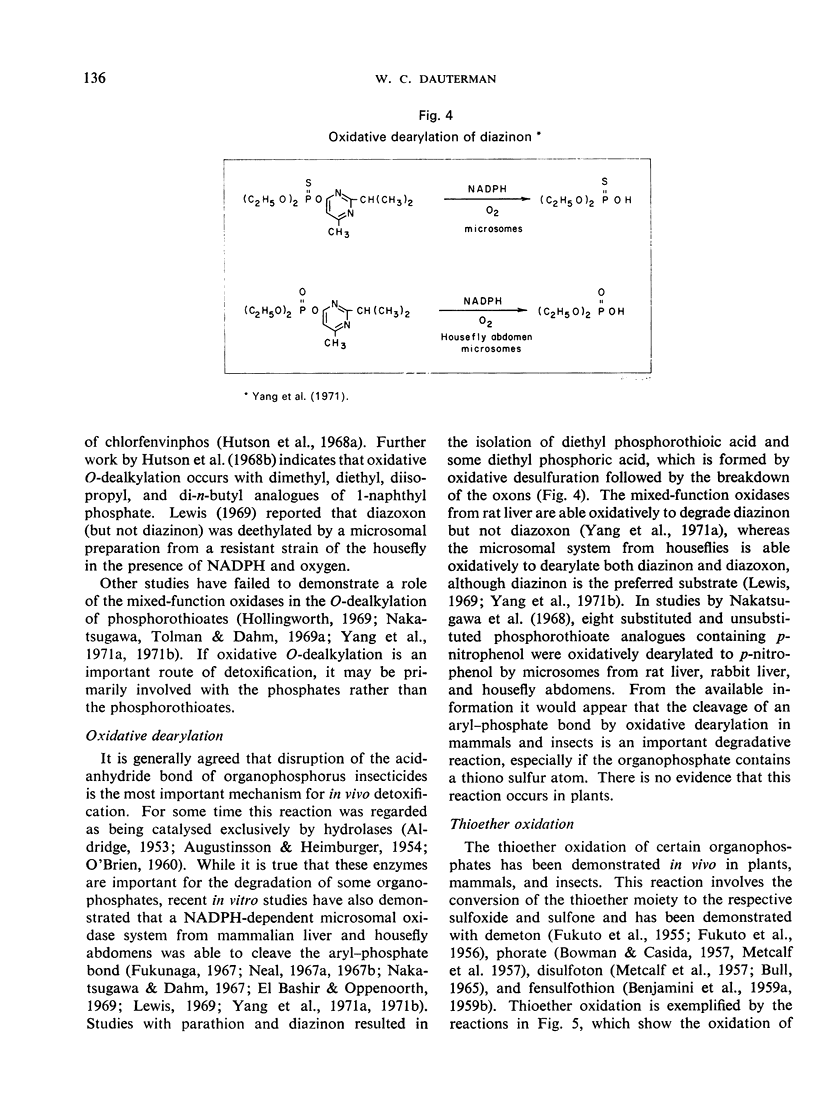
- Hutson D. H., Akintonwa D. A., Hathway D. E. The metabolism of 2-chloro-1-(2',4'-dichlorophenyl)vinyl diethyl phosphate (Chlorfenvinphos) in the dog and rat. Biochem J. 1967 Jan;102(1):133–142. doi: 10.1042/bj1020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaak J. B. Biological and nonbiological modifications of carbamates. Bull World Health Organ. 1971;44(1-3):121–131. [PMC free article] [PubMed] [Google Scholar]
- Lee Y. C., Hayes M. G., McCormicck D. B. Microsomal oxidation of alpha-thiocarboxylic acids to sulfoxides. Biochem Pharmacol. 1970 Nov;19(11):2825–2832. doi: 10.1016/0006-2952(70)90021-3. [DOI] [PubMed] [Google Scholar]
- Lewis J. B. Detoxification of diazinon by subcellular fractions of diazinon-resistant and susceptible houseflies. Nature. 1969 Nov 29;224(5222):917–918. doi: 10.1038/224917a0. [DOI] [PubMed] [Google Scholar]
- Lucier G. W., Menzer R. E. Nature of oxidative metabolites of dimethoate formed in rats, liver microsomes, and bean plants. J Agric Food Chem. 1970 Jul-Aug;18(4):698–704. doi: 10.1021/jf60170a034. [DOI] [PubMed] [Google Scholar]
- Lykken L., Casida J. E. Metabolism of organic insecticide chemicals. Can Med Assoc J. 1969 Jan 25;100(4):145–154. [PMC free article] [PubMed] [Google Scholar]
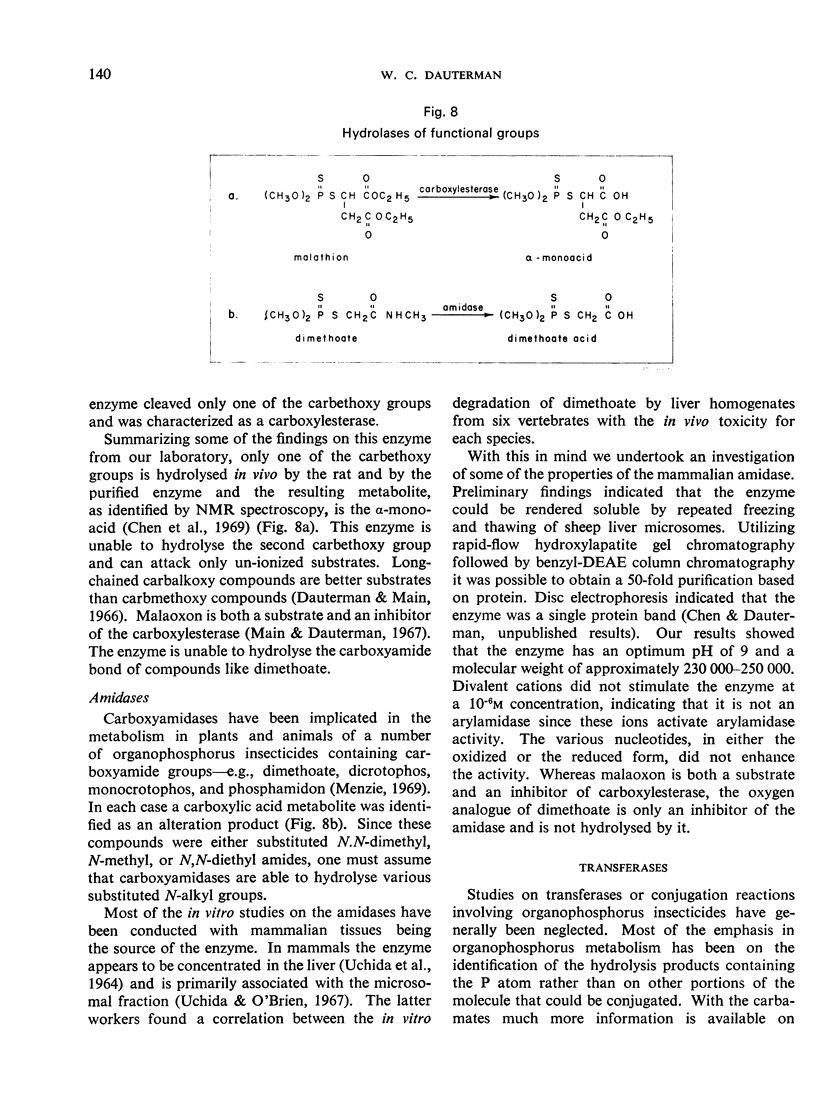
- MAIN A. R., BRAID P. E. Hydrolysis of malathion by ali-esterases in vitro and in vivo. Biochem J. 1962 Aug;84:255–263. doi: 10.1042/bj0840255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIN A. R. The differentiation of the A-type esterases in sheep serum. Biochem J. 1960 Apr;75:188–195. doi: 10.1042/bj0750188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIN A. R. The purification of the enzyme hydrolysing diethyl p-nitrophenyl phosphate (paraoxon) in sheep serum. Biochem J. 1960 Jan;74:10–20. doi: 10.1042/bj0740010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY S. D., DUBOIS K. P. Quantitative measurement of inhibition of the enzymatic detoxification of malathion by EPN (ethyl p-nitrophenyl thionobenzenephosphonate). Proc Soc Exp Biol Med. 1957 Dec;96(3):813–818. doi: 10.3181/00379727-96-23617. [DOI] [PubMed] [Google Scholar]
- Main A. R., Dauterman W. C. Kinetics for the inhibition of carboxylesterase by malaoxon. Can J Biochem. 1967 Jun;45(6):757–771. doi: 10.1139/o67-087. [DOI] [PubMed] [Google Scholar]
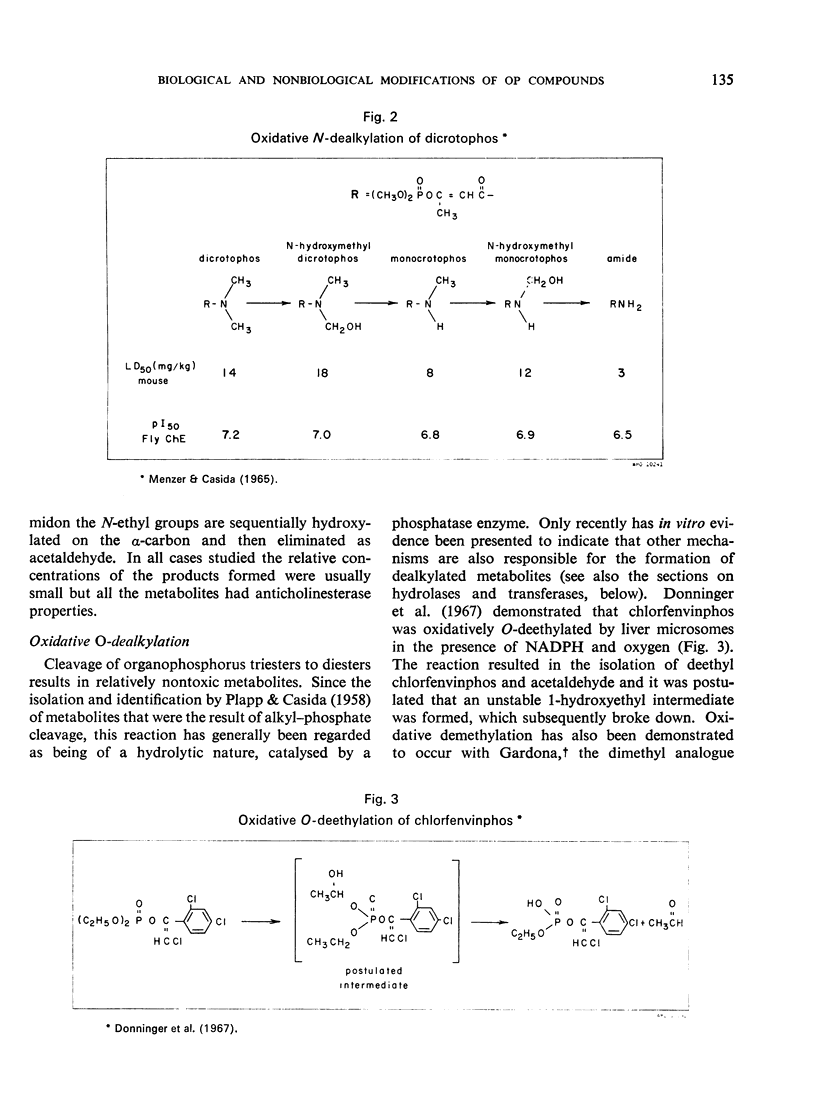
- Menzer R. E., Dauterman W. C. Metabolism of some organophosphorus insecticides. J Agric Food Chem. 1970 Nov-Dec;18(6):1031–1037. doi: 10.1021/jf60172a043. [DOI] [PubMed] [Google Scholar]
- Mitchell T. H., Ruzicka J. H., Thomson J., Wheals B. B. The chromatographic determination of organophosphorus pesticides. 2. The effect of irradiation on the parent compounds. J Chromatogr. 1968 Jan 9;32(1):17–23. doi: 10.1016/s0021-9673(01)80468-4. [DOI] [PubMed] [Google Scholar]
- Morello A., Vardanis A., Spencer E. Y. Mechanism of detoxication of some organophosphorus compounds: the role of glutathione-dependent demethylation. Can J Biochem. 1968 Aug;46(8):885–892. doi: 10.1139/o68-132. [DOI] [PubMed] [Google Scholar]
- Mücke W., Alt K. O., Esser H. O. Degradation of 14 C-labeled Diazinon in the rat. J Agric Food Chem. 1970 Mar-Apr;18(2):208–212. doi: 10.1021/jf60168a020. [DOI] [PubMed] [Google Scholar]
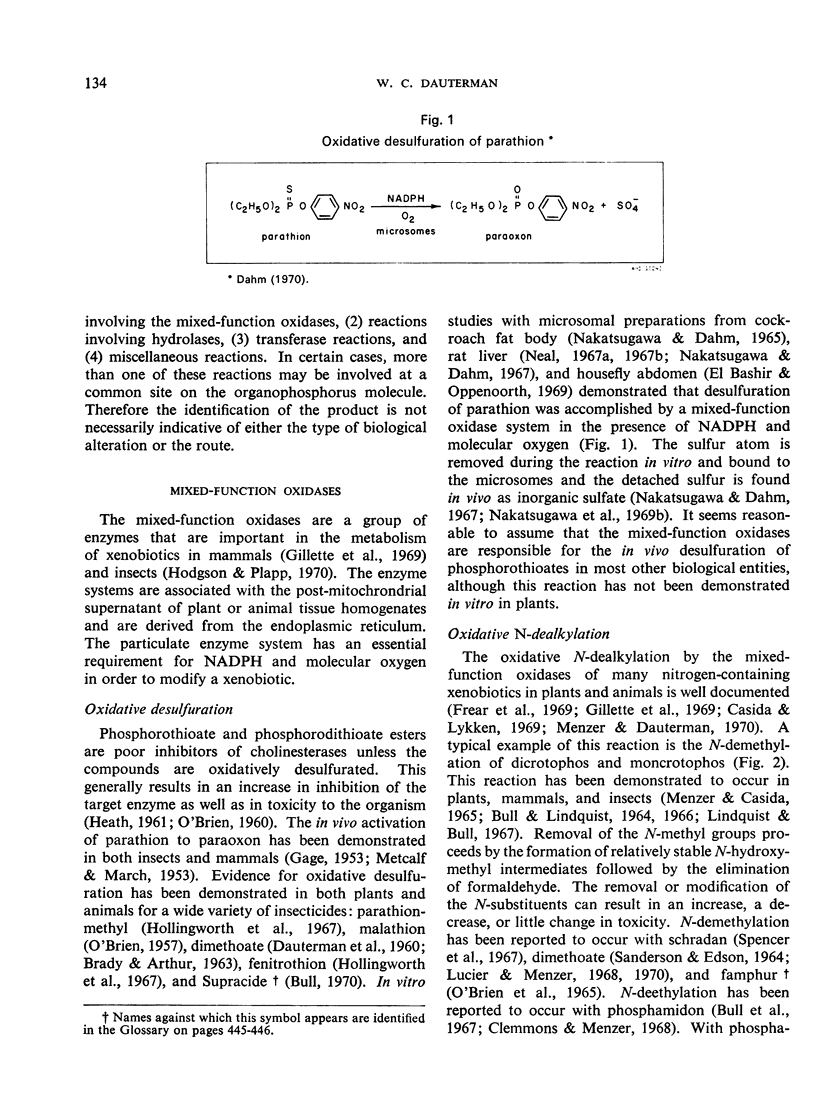
- NAKATSUGAWA T., DAHM P. A. PARATHION ACTIVATION ENZYMES IN THE FAT BODY MICROSOMES OF THE AMERICAN COCKROACH. J Econ Entomol. 1965 Jun;58:500–509. doi: 10.1093/jee/58.3.500. [DOI] [PubMed] [Google Scholar]
- Nakatsugawa T., Tolman N. M., Dahm P. A. Degradation and activation of parathion analogs by microsomal enzymes. Biochem Pharmacol. 1968 Aug;17(8):1517–1528. [PubMed] [Google Scholar]
- Nakatsugawa T., Tolman N. M., Dahm P. A. Degradation of parathion in the rat. Biochem Pharmacol. 1969 May;18(5):1103–1114. doi: 10.1016/0006-2952(69)90114-2. [DOI] [PubMed] [Google Scholar]
- Nakatsugawa T., Tolman N. M., Dahm P. A. Oxidative degradation of diazinon by rat liver microsomes. Biochem Pharmacol. 1969 Mar;18(3):685–688. doi: 10.1016/0006-2952(69)90096-3. [DOI] [PubMed] [Google Scholar]
- Neal R. A. Studies of the enzymic mechanism of the metabolism of diethyl 4-nitrophenyl phosphorothionate (parathion) by rat liver microsomes. Biochem J. 1967 Oct;105(1):289–297. doi: 10.1042/bj1050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal R. A. Studies on the metabolism of diethyl 4-nitrophenyl phosphorothionate (parathion) in vitro. Biochem J. 1967 Apr;103(1):183–191. doi: 10.1042/bj1030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYTON J. Parathion and ultra-violet light. Nature. 1953 Feb 21;171(4347):355–356. doi: 10.1038/171355a0. [DOI] [PubMed] [Google Scholar]
- Pardue J. R., Hansen E. A., Barron R. P., Chen J. Y. Diazinon residues on field-sprayed kale hydroxydiazinon--a new alteration product of diazinon. J Agric Food Chem. 1970 May-Jun;18(3):405–408. doi: 10.1021/jf60169a031. [DOI] [PubMed] [Google Scholar]
- SANDERSON D. M., EDSON E. F. TOXICOLOGIC PROPERTIES OF THE ORGANOPHOSPHORUS INSECTICIDE DIMETHOATE. Br J Ind Med. 1964 Jan;21:52–64. doi: 10.1136/oem.21.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenersen J. Demethylation of the insecticide bromophos by a glutathione-dependent liver enzyme and by alkaline buffers. J Econ Entomol. 1969 Oct;62(5):1043–1045. doi: 10.1093/jee/62.5.1043. [DOI] [PubMed] [Google Scholar]
- Tsukamoto M., Casida J. E. Albumin enhancement of oxidative metabolism of methylcarbamate insecticide chemicals by the house fly microsome-NADPH2 system. J Econ Entomol. 1967 Apr;60(2):617–619. doi: 10.1093/jee/60.2.617. [DOI] [PubMed] [Google Scholar]
- Uchida T., O'Brien R. D. Dimethoate degradation by human liver and its significance for acute toxicity. Toxicol Appl Pharmacol. 1967 Jan;10(1):89–94. doi: 10.1016/0041-008x(67)90131-7. [DOI] [PubMed] [Google Scholar]
- VANDEKAR M., HEATH D. F. The reactivation of cholinesterase after Inhibition in vivo by some dimethyl phosphate esters. Biochem J. 1957 Oct;67(2):202–208. doi: 10.1042/bj0670202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF S. A importância do stress na úlcera péptica. Resen Clin Cient. 1956 Jun;25(6):147–151. [PubMed] [Google Scholar]
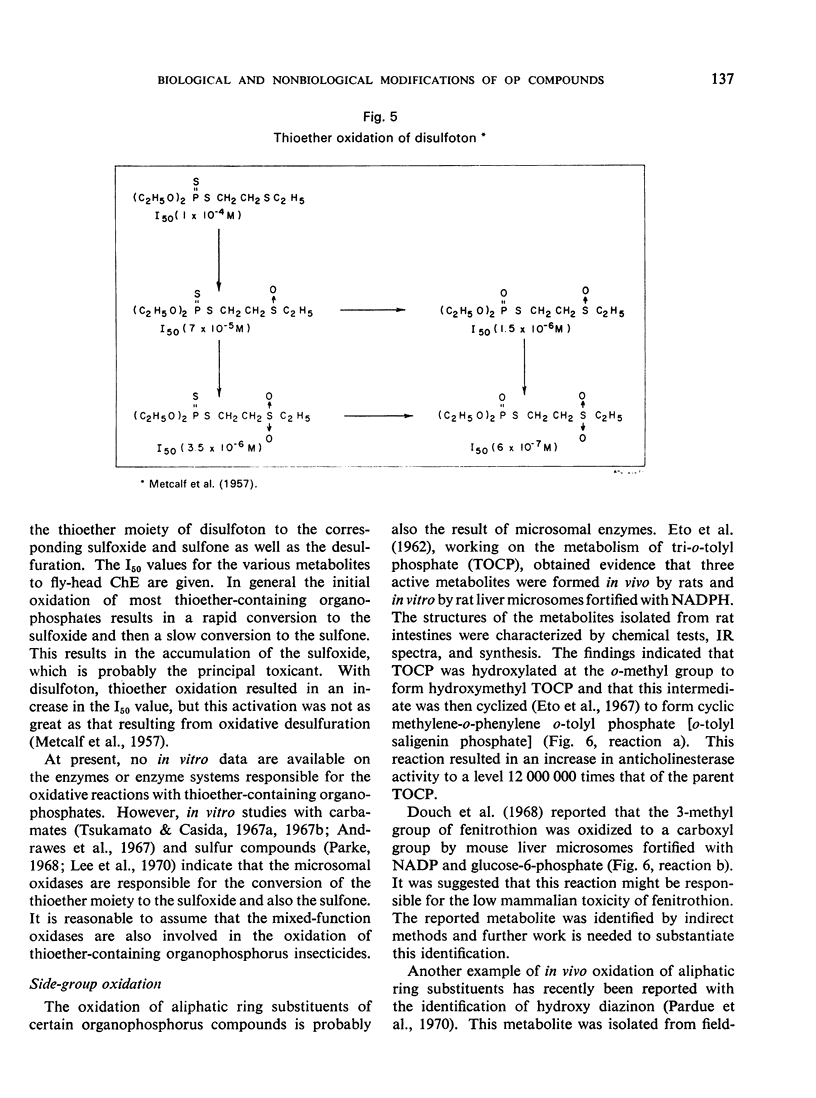
- Yang R. S., Dauterman W. C., Hodgson E. Enzymatic degradation of diazinon by rat liver microsomes. Life Sci. 1969 Jun 1;8(11):667–672. doi: 10.1016/0024-3205(69)90028-9. [DOI] [PubMed] [Google Scholar]
- Yang R. S., Hodgson E., Dauterman W. C. Metabolism in vitro of diazinon and diazoxon in rat liver. J Agric Food Chem. 1971 Jan-Feb;19(1):10–13. doi: 10.1021/jf60173a040. [DOI] [PubMed] [Google Scholar]
- el Bashir S., Oppenoorth F. J. Microsomal oxidations of organophosphate insecticides in some resistant strains of houseflies. Nature. 1969 Jul 12;223(5202):210–211. doi: 10.1038/223210a0. [DOI] [PubMed] [Google Scholar]


