Abstract
Matrix metalloproteineases are associated with extracellular remodeling that occurs in injury and repair processes in the central nervous system (CNS). We examined the role of MMP-2 in a model of olfactory nerve injury and found that MMP-2 levels increased several hours following injury, peaked at day 7 and then decreased rapidly. We previously reported a rapid increase in MMP-9, within 5 h after nerve injury, corresponding to neuronal degeneration and increased glial activity. In this study, we show that MMP-2 peaks later than MMP-9, at the onset of neuronal regeneration and repair. Using MMP-9 knockout mice, we determined that the MMP-2 increase is independent of MMP-9. Our data suggest that MMP-2 and MMP-9 may play different roles in the injury and repair processes.
Keywords: central nervous system, degeneration, matrix metalloproteinase-2, nerve injury, olfactory bulb
Introduction
Matrix metaloproteinases (MMPs) are a family of proteinases that function to cleave virtually all components of the extracellular matrix (ECM), making them excellent mediators of early inflammatory processes, tissue remodeling and scar formation following a variety of injury types. In particular, the gelatinases, MMP-2 (gelatinase A) and MMP-9 (gelatinase B) degrade common ECM components such as types IV and V collagen, fibronectin, elastin, and laminin, as well as the major central nervous system (CNS) matrix component, chondroitin sulfate proteoglycans (CSPGs). Interestingly, some MMP-2 and MMP-9 substrates are also components of the endothelial basement membrane in cerebral vasculature. Therefore, it is not surprising that the gelatinases have been implicated in numerous processes following different types of injury in the CNS, including ischemia, traumatic brain injury and spinal cord injury [1–5]. MMP-2 and MMP-9 have been linked to blood–brain barrier disruption, inflammation, angiogenesis, remodeling of the ECM and glial scar formation [3,6–9].
Although both MMP-2 and MMP-9 have been linked to processes following injury in the CNS, evidence suggests that the two gelatinases may play different roles in events surrounding CNS injury and recovery. Their action on specific substrates, differences in localization within the CNS, and differences in temporal expression suggest that the two gelatinases perform different functions. Specifically in the CNS, MMP-9 is often elevated rapidly following injury, particularly during the early stages of inflammation and blood–brain barrier disruption [1,10]. Elevations in MMP-2 levels are often observed later, after the initial, acute injury response [7,11,12].
We previously used the olfactory system as an injury model to examine the role of MMP-9 following olfactory nerve injury, specifically in the processes associated with olfactory neuron degeneration and regeneration. The olfactory system provides a unique model for neuronal injury and repairs as olfactory neurons are continuously replaced over the entire lifespan and have the capacity to regenerate and reestablish connections with the olfactory bulb following injury. A well-defined time course of events following olfactory nerve injury has been established [13]. Degeneration of sensory neurons occurs over the first few days following neuronal injury and is followed by regeneration of cells in the epithelium, reestablishing connections to the olfactory bulb over a 10 to 40-day time period. Following neuronal injury, extensive remodeling of the ECM would be required in order for successful recovery of functional connections with the olfactory bulb. We previously showed that following olfactory nerve injury, MMP-9's rapid and early elevation for 1−10 days post injury was temporally associated with the period of neuronal degeneration and the onset of gliosis. Given MMP-9's early modulation following nerve injury and the amount of time required to fully restore connections to the olfactory bulb, we hypothesized MMP-2 would also be modulated in the olfactory bulb. Considering MMP-9's rapid and early onset, however, it seems likely that MMP-2 would be associated with recovery processes, that occur later, following peak MMP-9 expression, and that MMP-2 expression would be independent of that of MMP-9. If confirmed this would suggest that MMP-9 and MMP-2 play different roles following olfactory nerve injury.
Methods
Surgical procedures
P2-IRES-tau-lacZ mice and MMP-9 Knockout (KO) mice (Jackson Laboratories, Bar Harbor, Maine, USA) were used in this study. Mice were anesthetized with (80 mg/kg, intraperitoneal) sodium pentobarbital, and bilateral olfactory nerve transections were performed as previously described [14]. The skull above the olfactory bulbs was removed, and the olfactory nerves transected by inserting a thin teflon cutting blade between each olfactory bulb and the cribiform plate. The teflon blade is designed to conform to the curved surface of the cribiform plate, resulting in only minimal damage to the surface of the bulb. Following nerve transection, the skin incision was sutured, the animal observed postoperatively during recovery from anesthesia and then returned to its home cage. Sham surgeries were also performed to control for effects of anesthesia and surgical exposure of the olfactory bulbs. All procedures were approved by Virginia Commonwealth University's Animal Care and Use Committee.
Tissue sampling and preparation
Following 5 h, 1,3,5,7,10,15,35, or 60 days of recovery, mice were anesthetized with sodium pentobarbital and sacrificed by rapid decapitation. The olfactory bulbs were removed from the skull and bisected, isolating the anterior–ventral portion of each bulb (the portion sustaining the most damage) for analysis. Olfactory bulbs were also obtained from control mice that did not undergo any surgical procedures. The anterior–ventral halves of both bulbs were then pooled and homogenized using a motor-driven plastic homogenizer in protein extraction buffer (50 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 1% sodium dodecyl sulfate, 1% Deoxycholic acid (DOC)) containing a protease inhibitor cocktail (Calbiochem, San Diego, California, USA). Tissue samples were incubated for 30 min on a rotating platform at 4°C, rehomogenized and centrifuged at 16 000g for 30 min at 4°C. Solubilized proteins in the supernatant were quantified with a BioRad DC protein assay kit (Bio-Rad Laboratories, Hercules, California, USA), using bovine serum albumin as a standard. Protein quantification was performed on an UQuant plate reader (BioTek Instruments Inc., Winooki, Vermont, USA) at 720 nm. Equal protein samples and purified mouse MMP-2 (R&D Systems, Minneapolis, Minnesota, USA) were loaded onto Bis–Tris 4−12% density gradient gels (Invitrogen, Carlsbad, California, USA) and separated using NuPAGE MES [2–(N-morpholino) ethane sulfonic acid] reducing buffer system at 180 V for 1.5 h and 4°C. Protein was then transferred to nitrocellulose membranes at 25 v for 2 h at 4°C. Following transfer, nonspecific binding was blocked by incubation of nitrocellulose membranes in 5% bovine, nonfat dry milk in TBS-T (Tris buffered saline and 0.05% Tween-20) for 1 h. Primary antibody against MMP-2 (1 : 200) was obtained from R&D Systems (Minneapolis, Minnesota, USA). Primary antibody against Cyclophilin-A (1 : 4000, Upstate, Lake Placid, New York, USA) was used to control for differences in protein loading. Nitrocellulose membranes were then incubated in primary antibody overnight at 4°C. Following incubation in primary antibody, membranes were incubated for 1 h in species-specific peroxidase-conjugated IgG (Jackson Immunochemicals, West Grove, Pennsylvania, USA) and treated for 1 min with Western Lightning Plus Chemiluminescent reagent (Perkin Elmer, Wellesley, Massachusetts, USA). Proteins were detected by exposure of membranes to Blue Sensitive Autoradiography film (Marsh BioProducts, Rochester, New York, USA).
Protein quantification
MMP-2 was quantified using Quantity One Analysis Software (Bio-Rad Laboratories, Hercules, California, USA). MMP-2 expression was measured as a function of both band density and area. The MMP-2 density-area measurement was then standardized against the density area measurement for Cyclophilin A (CPA) in the same gel. The amount of MMP-2 was then expressed as the ratio of MMP-2 to CPA.
Results
MMP-2 protein expression was measured shortly after olfactory nerve transection and during degeneration and regeneration time periods. The data from Western blots illustrating the time course of MMP-2 expression from P2 mice are shown in Fig. 1. Density-area measurements of MMP-2 bands were compared with those for CPA (Fig. 1a) to determine the relative amount of MMP-2 at each time point during recovery. Time course data from four separate experiments are plotted in Fig. 1b. In control mice, MMP-2 levels were barely detectable or not present. MMP-2 levels increased within 5 h following injury and increased slowly through day 3. MMP-2 levels increased rapidly and reached a maximum peak level at day 7 in all but one experiment. At their maximum, MMP-2 levels reached 5 to 20-fold those observed at the earliest time point, 5 h, following injury. In one experiment, the rapid increase was observed between days 3 and 10 and the peak level at day 10. Following maximum expression, MMP-2 levels rapidly declined, returning to baseline levels by day 15. MMP-2 levels remained at these low levels for the remaining 60-day recovery period. Samples were also obtained from several mice at days 3, 7 and 10 following sham surgeries (bulbs surgically exposed but nerve transection not performed). In these mice, MMP-2 was present but the levels were barely detectable (data not shown), a finding similar to that observed for the control mice.
Fig. 1.
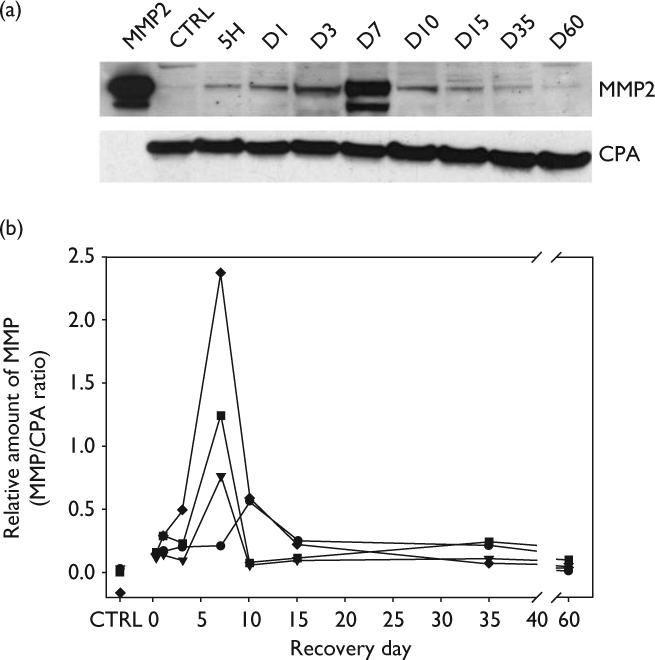
Analysis of MMP-2 expression in the olfactory bulb following olfactory nerve transection injury in P2 mice. (a) Representative Western blot illustrating changes in MMP-2 expression at different time points following injury. Lane 1 shows pro (upper band) and active forms (lower band) of purified murine MMP-2 standard. Lane 2 shows that MMP-2 is barely detectable in control mice (CTRL). Cyclophilin A (CPA) expression at each time point was used to adjust for differences in protein loading. (b) Plot of the relative amounts of MMP-2 (pro and active forms included in the quantification) from four separate experiments expressed as percentages of CPA. Each experiment represents data from nine separate mice. MMP-2 levels initially increased slowly, rose rapidly after day 3 to a maximum at day 7. MMP-2 levels declined rapidly between day 7 and day 10. CPA, Cyclophilin A; MMP, matrix metalloproteinases.
MMP-2 protein expression following olfactory nerve transection was also measured in MMP-9 KO mice. Western blot analysis of MMP-2 expression in the MMP-9 KO mice is shown in Fig. 2. Density area measurements of MMP-2 bands were compared with those for CPA (Fig. 2a) to calculate a relative amount of MMP-2 at each time point during recovery. Data from four separate experiments are plotted in Fig. 2b. In control mice, MMP-2 levels were barely detectable. Small increases in MMP-2 levels, however, were observed on day 1 following injury. Although MMP-2 increased early reaching a maximum at day 7, the most dramatic increase in MMP-2 levels was observed between days 3 and 7. At its peak at day 7, the level of MMP-2 increased approximately 5 to 20-fold compared with levels measured at day 1. Following the sharp rise during the early time points through the day 7 maximum, MMP-2 levels fell rapidly through days 10−15. The MMP-2 levels present during the latter portion of the regeneration phase (days 15−30) were similar to those expressed at the early recovery time point (day 1). Samples from sham surgical mice at days 3, 7 and 10 had barely detectable levels of MMP-2 (data not shown).
Fig. 2.
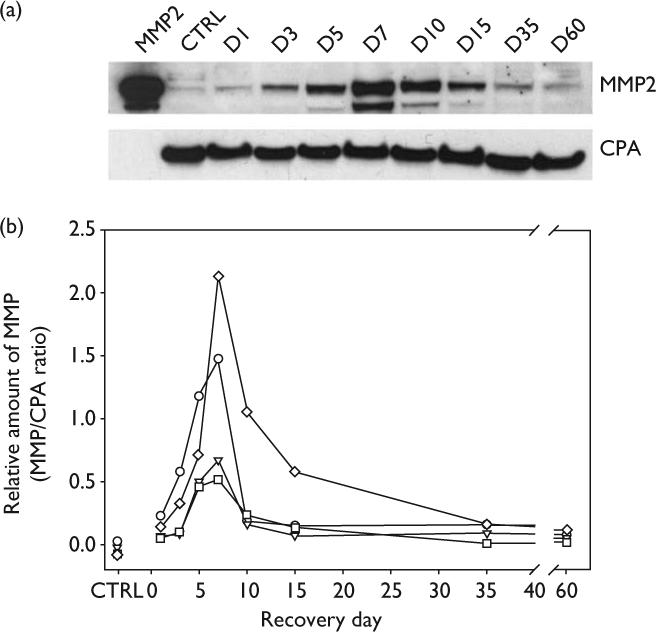
Analysis of MMP-2 expression in the olfactory bulb following olfactory nerve transection injury in MMP-9 KO mice. (a) Representative Western blot illustrating changes in MMP-2 expression at different time points following injury. Lane 1 shows pro and active forms of purified murine MMP-2 standard. Lane 2 shows that MMP-2 is barely detectable in control mice (CTRL). Cyclophilin A (CPA) expression at each time point was used to adjust for differences in protein loading. (b) Plot of the relative amounts of MMP-2 (pro and active forms included in the quantification) from four separate experiments expressed as percentages of CPA. Each experiment represents data from nine separate mice. MMP-2 levels initially increased slowly, rose rapidly after day 3 to a maximum at day 7. MMP-2 levels declined equally as rapidly from day 7 to day 10 or 15. Elevation in the level of MMP-2 in the absence of MMP-9 (MMP-9 KO mice) illustrates that MMP-9 is not required for the increase in MMP-2 expression following olfactory nerve injury. KO, Knockout; MMP, matrix metaloproteinases.
Figure 3 compares the time course of MMP-2 and MMP-9 expression in the olfactory bulb following olfactory nerve transection injury. MMP-2 levels in both the MMP-9 KO mice and the wild-type P2 mice showed nearly identical changes in the temporal expression of MMP-2. MMP-2 levels initially increased slowly following injury, then rapidly peaked reaching its maximum level at day 7. In contrast, MMP-9 levels in the wild-type P2 mice increased rapidly following injury, reaching its maximum at day 1 [14]. Although MMP-2 levels in both P2 mice and MMP-9 KO mice decreased rapidly after achieving peak levels at day 7, MMP-9 levels in P2 mice remained elevated for 2 weeks following injury, returning to near control levels later in the recovery period. Although the increase in MMP-9 levels always preceded the MMP-2 increase, the MMP-2 increase was still observed even in the absence of MMP-9 (MMP-9 KO mice).
Fig. 3.
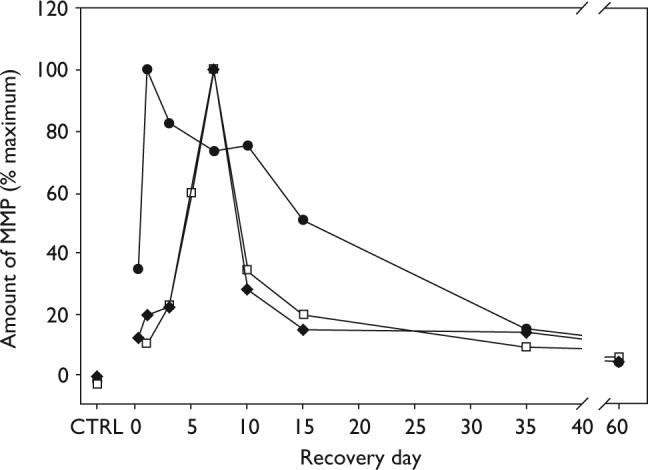
Summary of time course differences in MMP-2 and MMP-9 expression in the olfactory bulbs of P2 and MMP-9 KO mice following olfactory nerve transection injury. The MMP-9 data (filled circles) is from NeuroReport 2006; 17:1787−1791. The average MMP/ CPA ratio from four separate time course experiments were calculated and plotted as a percent of the maximum value. In P2 mice, MMP-9 peaked very rapidly (hours) after injury and remained elevated during olfactory neuron regeneration The MMP-2 expression data for both the P2 mice (filled diamonds) and MMP-9 KO mice (open squares) show nearly identical changes in the time course of expression following injury. MMP-2 levels peaked later than MMP-9, at day 7 and returned rapidly to the lower levels observed immediately following injury. KO, Knockout; MMP, matrix metaloproteinases.
Discussion
Recovery from injury in the CNS is marked by extensive extracellular remodeling. In particular, the two gelatinases, MMP-2 and MMP-9, have been the focus of studies in CNS injury models because of their abilities to degrade prominent components of the ECM following injury [15]. The components of scar in the CNS are well known for their inhibition of successful repair of CNS connections. Injury in the CNS results in ECM remodeling even in cases where there is no neuronal regeneration. Neuronal regeneration following CNS injury, however, requires a more complex and regulated ECM remodeling, given that axons must regrow not only over long distances but also through physical and chemical barriers to reach their targets. We previously reported elevations in MMP-9 immediately following olfactory nerve injury, suggesting a correlation with inflammation, disruption of the blood–brain barrier and neuronal degeneration, all events associated with rapid onset postinjury processes. In this study, elevations of MMP-2 were observed later in the recovery period than those observed for MMP-9, suggesting a different role for MMP-2.
Other CNS injury studies have reported MMP-2 elevation within a few hours to 1 day after injury. As shown in this study, this initial increase, however, was followed days later by a peak in MMP-2 levels peaked at about 1 week following injury [3,4,11,12,16]. The delayed onset of the MMP-2 increases following CNS injury and its specific ability to degrade collagens, and CSPGs, central components of scar in the CNS following injury, suggest that MMP-2's role may be to promote axonal regeneration and elongation. CSPGs, in particular, are a family of inhibitory ECM molecules derived from several components of scar, oligodendrocytes, reactive astrocytes, fibroblasts and microglia, in the CNS following injury [17]. The CSPGs produced in CNS scar not only contribute to a physical scar barrier, but also are an inhibitory chemical barrier at the injury site. These barriers may inhibit or arrest regenerating axons, even causing growth cone collapse [17,18]. MMP-2 could help support axonal regeneration as a result of CSPG degradation in the maturing scar. When ECM in scar is degraded, axon regeneration across the injury area is often promoted. Injections of chondroitinases into CNS injury sites result in significant axonal regeneration, even through scar [19–21]. MMP-2 shares similar CSPG degrading ability [16] and could be utilized to promote axonal regrowth following CNS injury. MMP-2 activity has been implicated in this scar remodeling as well as in axon outgrowth, even into the lesion following CNS injury [16,22]. Additionally, following spinal cord injury in the absence of MMP-2, astrocytic scar is more extensive [16].
Rapid onset processes such as inflammation, blood–brain barrier breakdown and even gliosis and neuronal degeneration occur within hours to just several days following injury and correlate with the very rapid MMP-9 elevation that has been previously observed following CNS injury [4,6,10], including recent observations in the olfactory system [14]. The onset of recovery and axonal regeneration processes occurs later. In this study, the modest increase in MMP-2 shortly following injury, followed by the dramatic rise between days 3 and 7, correlate well with the timing of astroglial scar maturation [3] and by the increase and maximum expression levels of GFAP [14]. In addition, MMP-2's maximum expression at day 7 following olfactory nerve injury also coincides with the conclusion of neuronal degeneration and the early onset of neuronal regeneration in the olfactory bulb [14]. This study shows a dramatic modulation of overall MMP-2 expression several days after olfactory nerve injury, and this modulation included substantial upregulation of the active form of MMP-2 from 3 to 10 days. This upregulation specifically of the active form also suggests that functional MMP-2 plays a role at a time when scar formation is most dramatic. In addition, the downregulation of MMP-2 over the remainder of the recovery time course following olfactory nerve injury also suggests a role in axonal regeneration through the scar barrier. Newly forming scars, within about 10 days of injury, are most likely better candidates for axon penetration and growth cone invasion [23] than mature scars with abundant fibroblast and astrocyte proliferation and copious inhibitory ECM.
MMP-2 and MMP-9 are both gelatinases with general substrate similarity, which might indicate a relationship or potential interdependence of function between the two. Following spinal cord injury, the absence of one gelatinase resulted in increased expression of the other [16], suggesting similar or complimentary roles. In our study, however, we observed increases in MMP-2 in the MMP-9 KO mice where MMP-9 was not present. Identical changes in MMP-2 expression with or without the presence of MMP-9 also suggest there may be two different roles for the gelatinases. MMP-9 appears to be associated with processes that occur during the initial response to injury whereas MMP-2 may play a role in later process associated with recovery.
Conclusion
This is the first report demonstrating that MMP-2 is associated with recovery events following olfactory nerve injury. The peak in MMP-2 levels observed at day 7 corresponds to a recovery period during which there is maximum regeneration of olfactory neurons and when axons first begin to reinnervate the olfactory bulb. The finding that MMP-2 also peaks at day 7 for those experiments performed in MMP-9 KO mice suggests that the MMP-2 increase is not dependent on increased levels of MMP-9 that begins immediately following olfactory nerve injury [14] and that the two play separate roles following olfactory nerve injury. Further studies are needed to investigate the mechanisms by which MMP-2 and MMP-9 might regulate injury and recovery processes. Interventions that modulate MMP levels could provide future therapeutic strategies to accelerate and improve recovery outcome following injury in the CNS.
Acknowledgements
We thank Dr Helen Fillmore for technical advice and suggestions during the project. This study was supported by Grant DC000165 from the National Institute on Deafness and Other Communication Disorders.
References
- 1.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 2.Lee SR, Tsuji K, Lee SR, Lo EH. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J Neurosci. 2004;24:671–678. doi: 10.1523/JNEUROSCI.4243-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goussev S, Hsu JY, Lin Y, Tjoa T, Maida N, Werb Z, et al. Differential temporal expression of matrix metalloproteinases after spinal cord injury: relationship to revascularization and wound healing. J Neurosurg Spine. 2003;99:188–197. doi: 10.3171/spi.2003.99.2.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Planas AM, Sole S, Justicia C. Expression and activation of matrix metalloproteinase-2 and -9 in rat brain after transient focal cerebral ischemia. Neurobiol Dis. 2001;8:834–846. doi: 10.1006/nbdi.2001.0435. [DOI] [PubMed] [Google Scholar]
- 5.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, et al. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 8.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 9.Hughes PM, Wells GM, Perry VH, Brown MC, Miller KM. Comparison of matrix metalloproteinase expression during Wallerian degeneration in the central and peripheral nervous systems. Neuroscience. 2002;113:273–287. doi: 10.1016/s0306-4522(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 10.Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg GA. Matrix metalloproteinases in brain injury. J Neurotrauma. 1995;12:833–842. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- 12.De Castro RC, Jr, Burns CL, McAdoo DJ, Romanic AM. Metalloproteinase increases in the injured rat spinal cord. NeuroReport. 2000;11:3551–3554. doi: 10.1097/00001756-200011090-00029. [DOI] [PubMed] [Google Scholar]
- 13.Costanzo RM. Neural regeneration and functional reconnection following olfactory nerve transection in hamster. Brain Res. 1985;361:258–266. doi: 10.1016/0006-8993(85)91297-1. [DOI] [PubMed] [Google Scholar]
- 14.Costanzo RM, Perrino LA, Kobayashi M. Response of matrix metalloproteinase-9 to olfactory nerve injury. NeuroReport. 2006;17:1787–1791. doi: 10.1097/WNR.0b013e32800fef87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 18.Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, et al. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100:103–117. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 19.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 20.Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- 21.Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, et al. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2004;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- 22.Duchossoy Y, Horvat JC, Stettler O. MMP-related gelatinase activity is strongly induced in scar tissue of injured adult spinal cord and forms pathways for ingrowing neurites. Mol Cell Neurosci. 2001;17:945–956. doi: 10.1006/mcne.2001.0986. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes KE, Moon LD, Fawcett JW. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120:41–56. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]


