Abstract
Nine strains of bacteroides fragilis were cultivated in stirred fermentors and tested for their ability to produce glycosidases. B. fragilis subsp. vulgatus B70 was used for optimizing the production of glycosidases. The highest bacterial yield was obtained in proteose peptone-yeast extract medium. The optimum pH for maximal bacterial yield was 7.0, and the optimum temperature for growth was 37 degrees C. The formation of glycosidases was optimal between pH 6.5 and 7.5, and the optimum temperature for synthesis of glycosidases was between 33 and 37 degrees C. Culture under controlled conditions in fermentors gave more reproducible production of glycosidases than static cultures in bottles. The strain was also grown in continuous culture at a dilution rate of 0.1 liter/h at pH 7.0 and 37 degrees C with a yield of 2.0 mg of dry weight per ml in the complex medium. The formation of glycosidases remained constant during the entire continuous process.
Full text
PDF

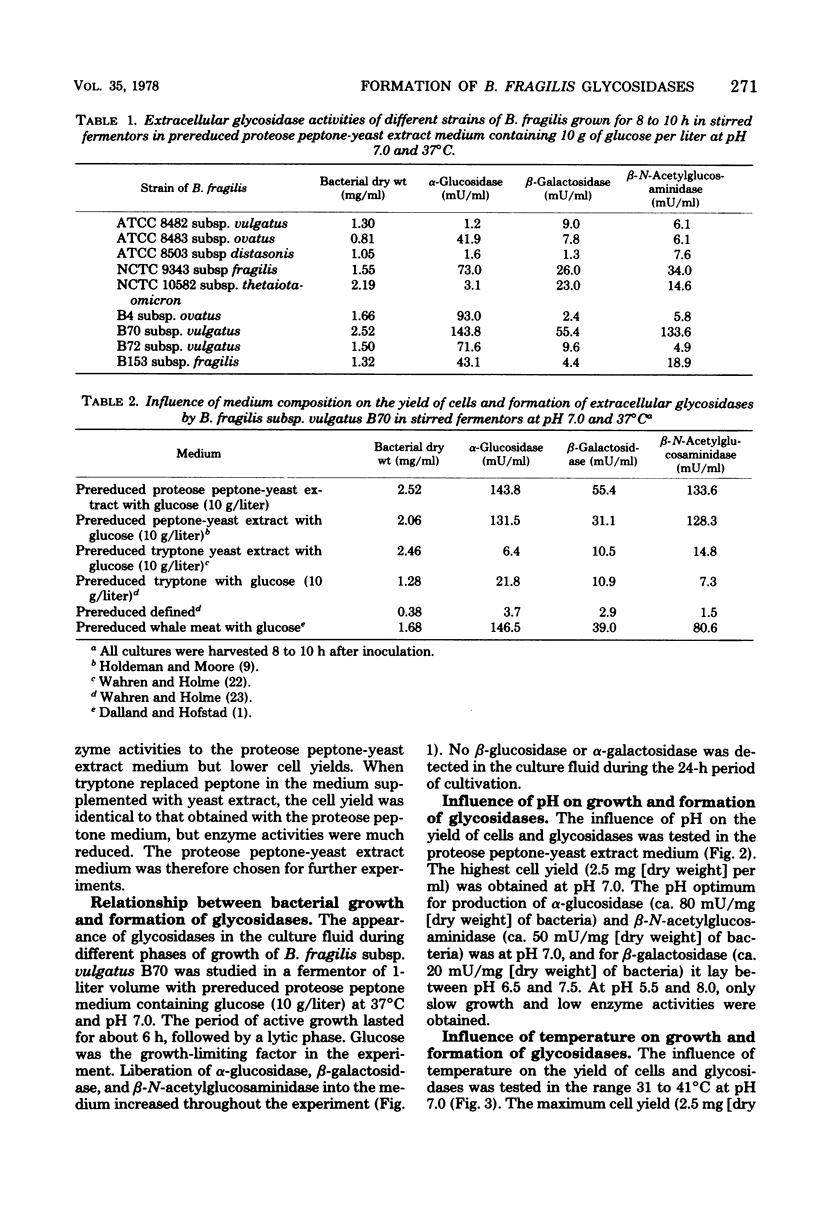

Selected References
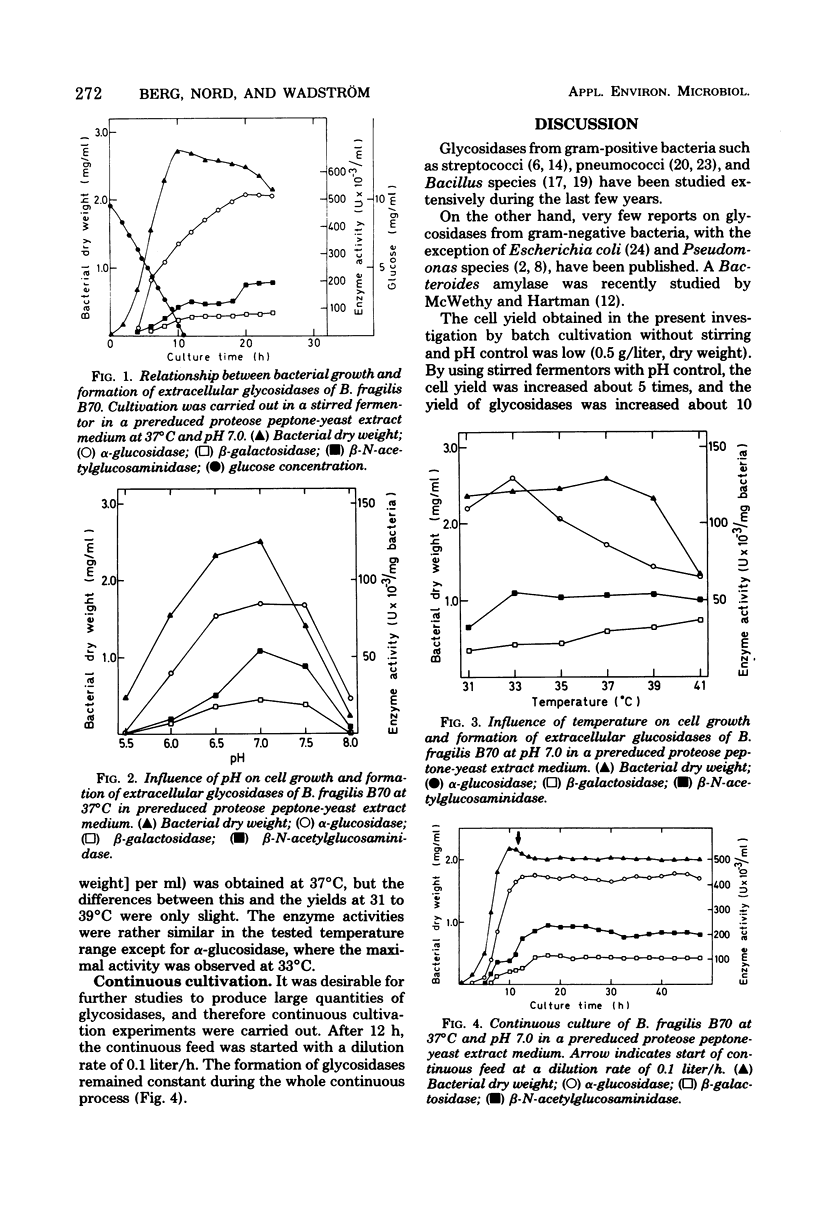
These references are in PubMed. This may not be the complete list of references from this article.
- Dalland E., Hofstad T. Growth of Bacteroides fragilis in continuous culture and in batch cultures at controlled pH. Appl Microbiol. 1974 Nov;28(5):856–860. doi: 10.1128/am.28.5.856-860.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. F., Gomersall M., Yaphe W. A p-nitrophenyl alpha-galactoside hydrolase from Pseudomonas atlantica. Localization of the enzyme. Can J Microbiol. 1975 Oct;21(10):1476–1483. doi: 10.1139/m75-219. [DOI] [PubMed] [Google Scholar]
- Desai P. D., Goldner M. Effect of low pH on thiomethyl-beta-D-galactoside uptake by Streptococcus lactis. J Bacteriol. 1969 Dec;100(3):1415–1416. doi: 10.1128/jb.100.3.1415-1416.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Corpe W. A. Partial purification and characterization of alpha-glucosidase from Pseudomonas fluorescens W. Arch Microbiol. 1976 Apr 1;107(3):269–276. doi: 10.1007/BF00425338. [DOI] [PubMed] [Google Scholar]
- Kilian M., Bülow P. Rapid diagnosis of Enterobacteriaceae. I. Detection of bacterial glycosidases. Acta Pathol Microbiol Scand B. 1976 Oct;84B(5):245–251. doi: 10.1111/j.1699-0463.1976.tb01933.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McWethy S. J., Hartman P. A. Purification and some properties of an extracellular alpha-amylase from Bacteroides amylophilus. J Bacteriol. 1977 Mar;129(3):1537–1544. doi: 10.1128/jb.129.3.1537-1544.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Linder L., Wadström T., Lindberg A. A. Formation of glycoside-hydrolases by oral streptococci. Arch Oral Biol. 1973 Mar;18(3):391–402. doi: 10.1016/0003-9969(73)90163-5. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Möllby R., Smyth C., Wadström T. Formation of phospholipase C and theta-haemolysin in pre-reduced media in batch anc continuous culture of Clostridium perfringens type A. J Gen Microbiol. 1974 Sep;84(1):117–127. doi: 10.1099/00221287-84-1-117. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Johnston J., Mayhew J. W., Gorbach S. L. Effect of dissolved oxygen and Eh and Bacteroides fragilis during continuous culture. Appl Environ Microbiol. 1976 Feb;31(2):168–172. doi: 10.1128/aem.31.2.168-172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J. M., Berkeley R. C., Brewer S. J. Production of exo-beta-N-acetylglucosaminidase by Bacillus subtilis B. J Gen Microbiol. 1973 Aug;77(2):331–337. doi: 10.1099/00221287-77-2-331. [DOI] [PubMed] [Google Scholar]
- Schmid K., Schmitt R. Raffinose metabolism in Escherichia coli K12. Purification and properties of a new alpha-galactosidase specified by a transmissible plasmid. Eur J Biochem. 1976 Aug 1;67(1):95–104. doi: 10.1111/j.1432-1033.1976.tb10637.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Kishigami T., Abe S. Production of extracellular alpha-glucosidase by a thermophilic Bacillus species. Appl Environ Microbiol. 1976 Jun;31(6):807–812. doi: 10.1128/aem.31.6.807-812.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. A comparison of the substrate specificities of endo-beta-N-acetylglucosaminidases from Streptomyces griseus and Diplococcus Pneumoniae. Biochem Biophys Res Commun. 1975 Nov 3;67(1):455–462. doi: 10.1016/0006-291x(75)90337-x. [DOI] [PubMed] [Google Scholar]
- WOOLLEN J. W., WALKER P. G., HEYWORTH R. Studies on glucosaminidase. 6. N-Acetyl-beta-glucosaminidase and N-acetyl-beta-galactosaminidase activities of a variety of enzyme preparations. Biochem J. 1961 May;79:294–298. doi: 10.1042/bj0790294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren A., Holme T. Amino acid and peptide requirement of Fusiformis necrophorus. J Bacteriol. 1973 Oct;116(1):279–284. doi: 10.1128/jb.116.1.279-284.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren A., Holme T. Growth of Bacteroidaceae in stirred fermentors. Appl Microbiol. 1969 Aug;18(2):235–239. doi: 10.1128/am.18.2.235-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. Purification and properties of beta-N-acetylglucosaminidase from Escherichia coli. J Bacteriol. 1976 Jan;125(1):324–331. doi: 10.1128/jb.125.1.324-331.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


