Abstract
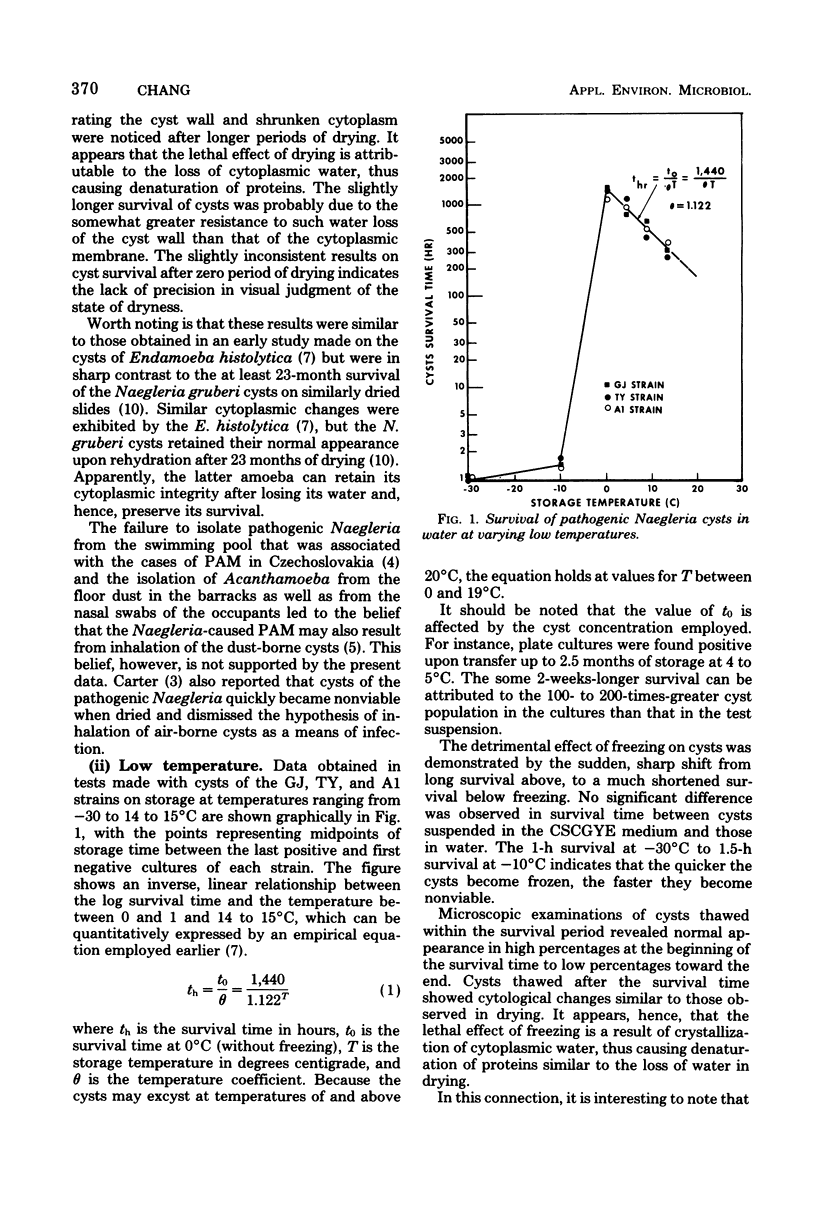
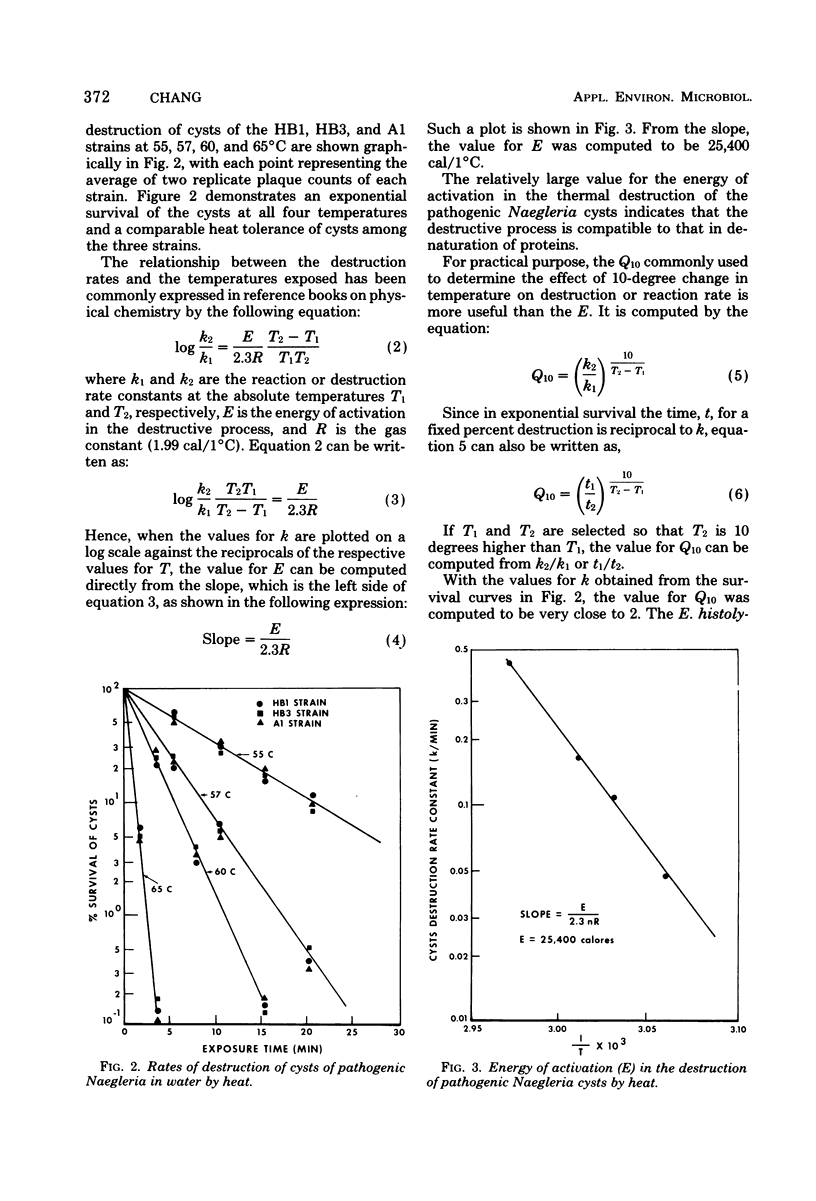
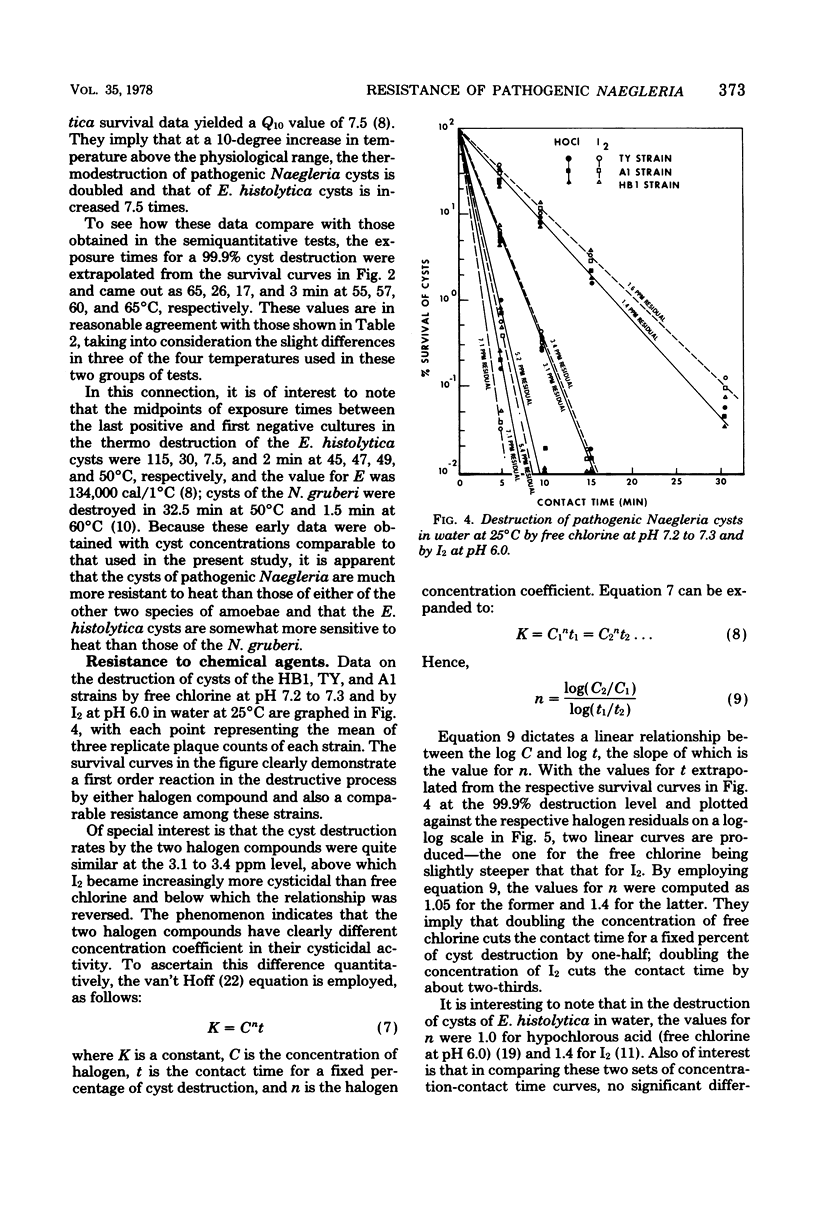
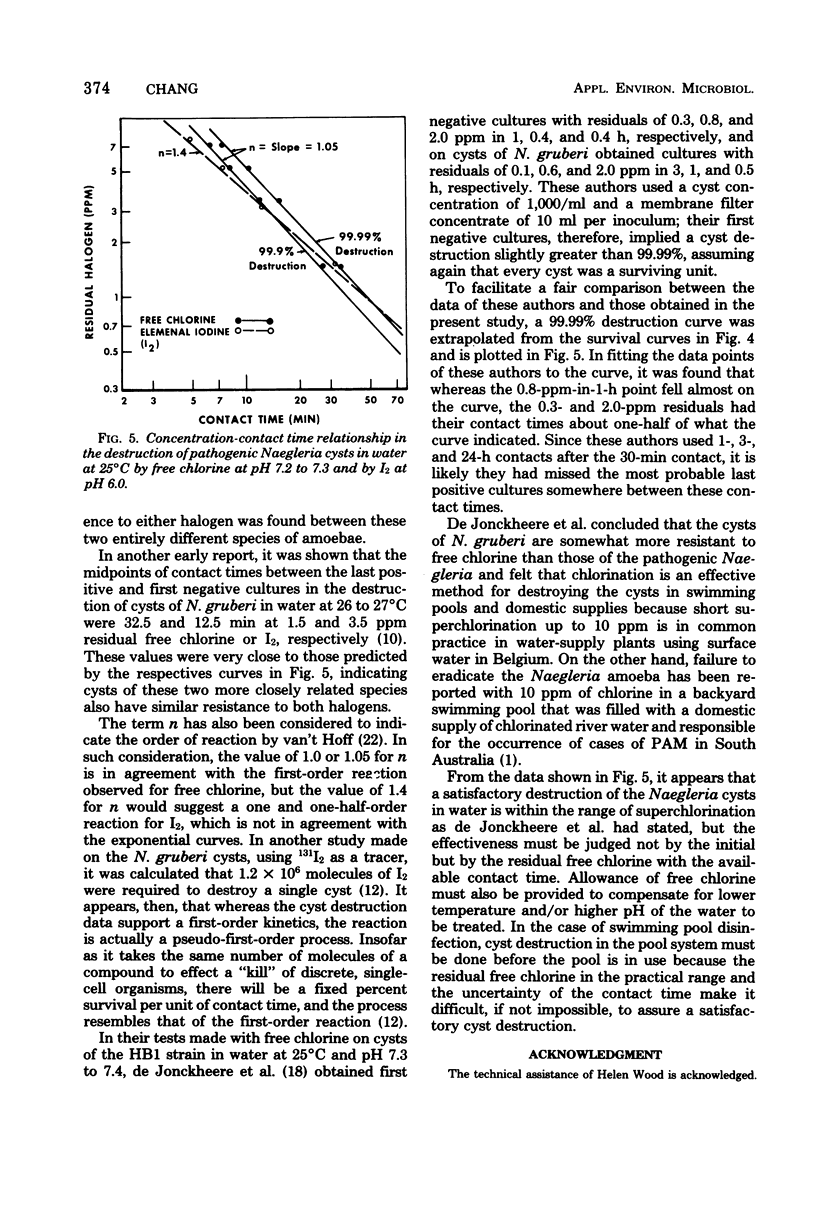
Resistance of pathogenic Naegleria to drying, low and high temperature, and two halogens was studied. Dying made trophozoites nonviable instantaneously and cysts nonviable in less than 5 min. Trophozoites degenerated in hours at temperatures below 10 degrees C and in minutes when frozen; cysts survived according to the equation th - t0/theta 1,440/1.122T (t0 is survival at 0 degrees C; Tis temperature between 0 and 10 degrees C), but 1.5 h at --10 degrees C to 1 h at --30 degrees C. At 51, 55, 58, 63, and 65 degrees C, trophozoites survived about 30, 10, 5, 1 and less than 0.5 min, respectively, cysts survived three to four times longer at 51 degrees C and six to seven times longer at 55 to 65 degrees C. Cyst destruction rates by heat indicated first-order kinetics with 25,400 cal/1 degree C for energy of activation. Cyst destruction rates by free chlorine and I2 also conformed to first-order kinetics. Concentration-contact time curves yielded concentration coefficient values of 1.05 for free chlorine and 1.4 for I2 and point to superchlorination as an effective means of destroying the cysts if free residuals are used as a guide and allowance is provided for low temperature and/or high pH waters.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Jamieson A. Primary amoebic meningoencephalitis. Lancet. 1972 Apr 22;1(7756):902–903. doi: 10.1016/s0140-6736(72)90772-6. [DOI] [PubMed] [Google Scholar]
- Butt C. G. Primary amebic meningoencephalitis. N Engl J Med. 1966 Jun 30;274(26):1473–1476. doi: 10.1056/NEJM196606302742605. [DOI] [PubMed] [Google Scholar]
- CHANG S. L., BERG G. Chlormelamine and iodized chlormelamine germicidal rinse formulations; essential physical and chemical characteristics, and germicidal efficiencies. U S Armed Forces Med J. 1959 Jan;10(1):33–49. [PubMed] [Google Scholar]
- CHANG S. L. Cultural, cytological and ecological observations on the amoeba stage of Naegleria gruberi. J Gen Microbiol. 1958 Jun;18(3):565–578. doi: 10.1099/00221287-18-3-565. [DOI] [PubMed] [Google Scholar]
- CHANG S. L. Kinetics in the thermodestruction of cysts of Endamoeba histolytica in water. Am J Hyg. 1950 Jul;52(1):82–90. doi: 10.1093/oxfordjournals.aje.a119411. [DOI] [PubMed] [Google Scholar]
- CHANG S. L. Survival of cysts of Endamoeba histolytica in human feces under low-temperature conditions. Am J Hyg. 1955 Jan;61(1):103–120. doi: 10.1093/oxfordjournals.aje.a119731. [DOI] [PubMed] [Google Scholar]
- CHANG S. L. The use of active iodine as a water disinfectant. J Am Pharm Assoc Am Pharm Assoc. 1958 Jun;47(6):417–423. [PubMed] [Google Scholar]
- Carter R. F. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J Pathol. 1970 Apr;100(4):217–244. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- Cerva L., Novák K., Culbertson C. G. An outbreak of acute, fatal amebic meningoencephalitis. Am J Epidemiol. 1968 Nov;88(3):436–444. doi: 10.1093/oxfordjournals.aje.a120905. [DOI] [PubMed] [Google Scholar]
- Cerva L., Serbus C., Skocil V. Isolation of limax amoebae from the nasal mucosa of man. Folia Parasitol (Praha) 1973;20(1):97–103. [PubMed] [Google Scholar]
- Chang S. L. Small, free-living amebas: cultivation, quantitation, identification, classification, pathogenesis, and resistance. Curr Top Comp Pathobiol. 1971;1:201–254. doi: 10.1016/b978-0-12-153401-1.50010-7. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J., Van Dijck P., Van de Voorde H. The effect of thermal pollution on the distribution of Naegleria fowleri. J Hyg (Lond) 1975 Aug;75(1):7–13. doi: 10.1017/s0022172400047021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonckheere J., van de Voorde H. Differences in destruction of cysts of pathogenic and nonpathogenic Naegleria and Acanthamoeba by chlorine. Appl Environ Microbiol. 1976 Feb;31(2):294–297. doi: 10.1128/aem.31.2.294-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. L. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science. 1972 Nov 24;178(4063):869–870. doi: 10.1126/science.178.4063.869. [DOI] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Amuso P. T., Chang S. L. Naegleria and water sports. Lancet. 1977 Jan 22;1(8004):199–200. doi: 10.1016/s0140-6736(77)91806-2. [DOI] [PubMed] [Google Scholar]
- de Jonckheere J., Voorde H. The distribution of Naegleria fowleri in man-made thermal waters. Am J Trop Med Hyg. 1977 Jan;26(1):10–15. doi: 10.4269/ajtmh.1977.26.10. [DOI] [PubMed] [Google Scholar]


