Abstract
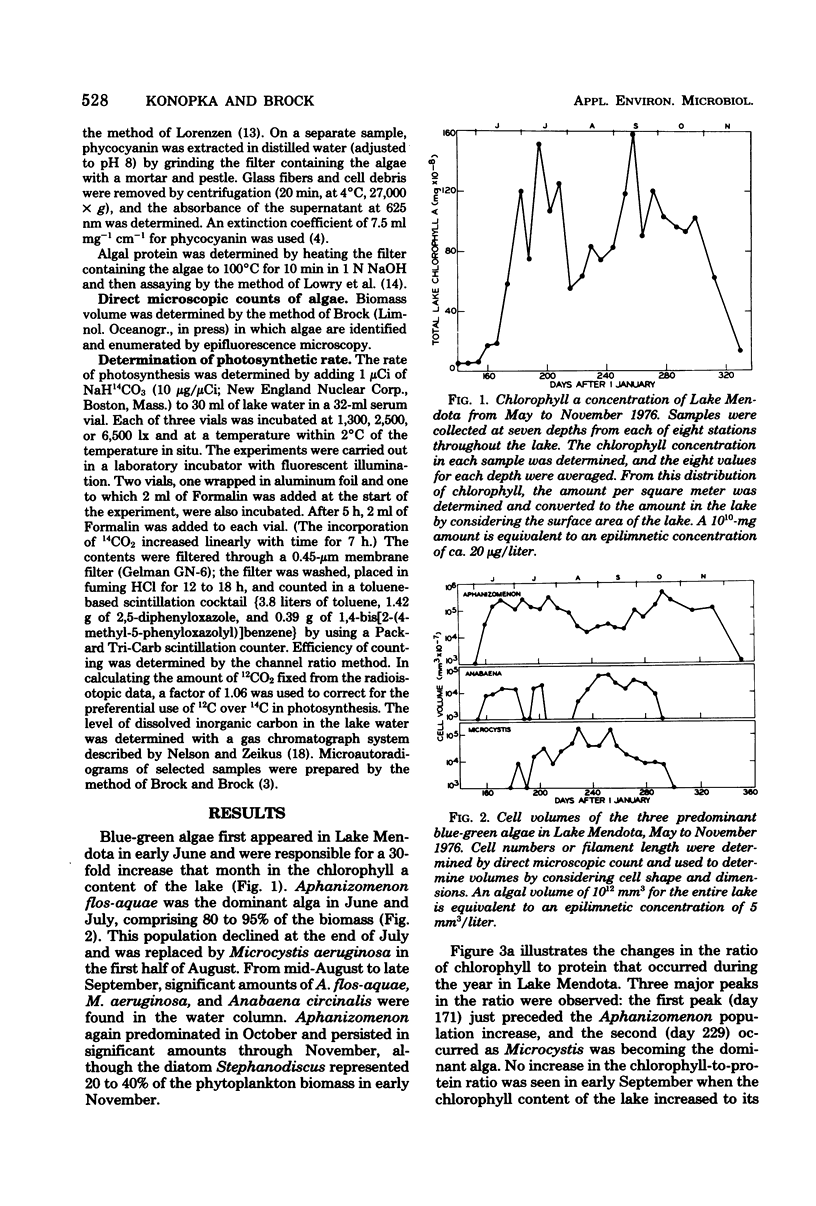
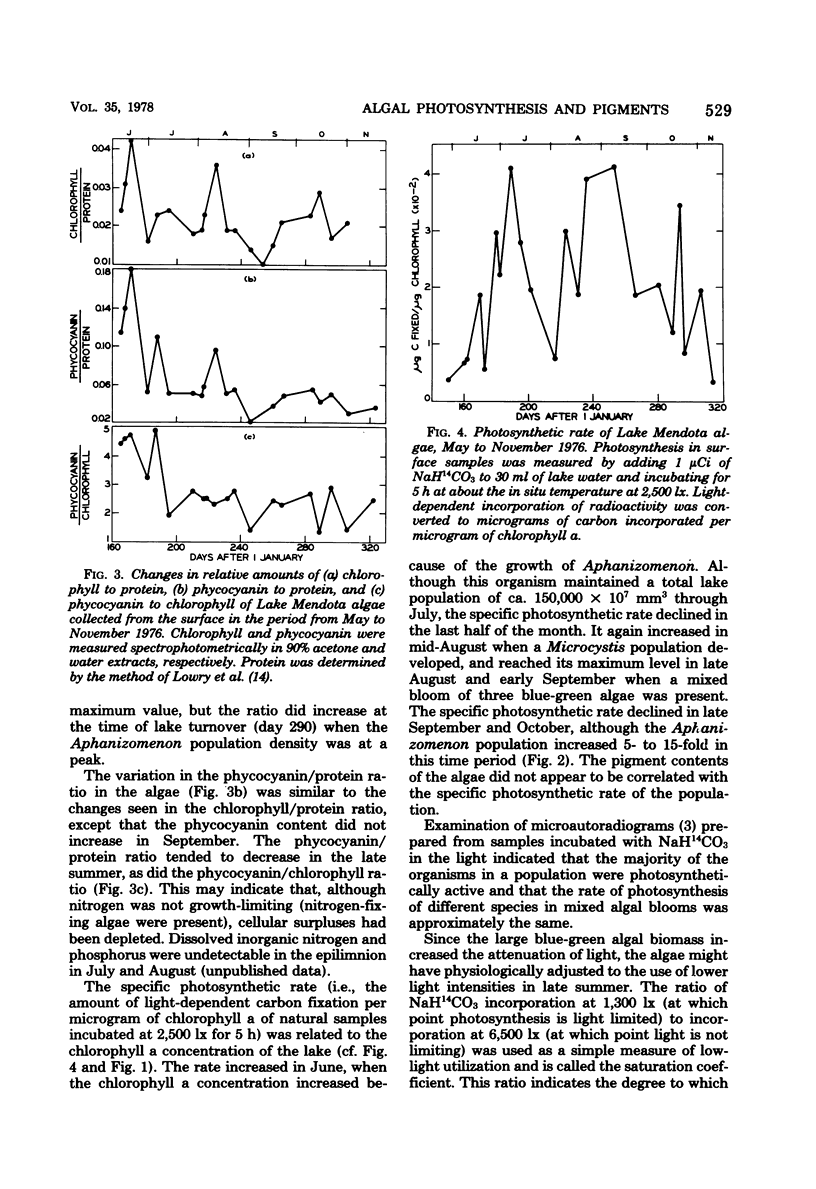
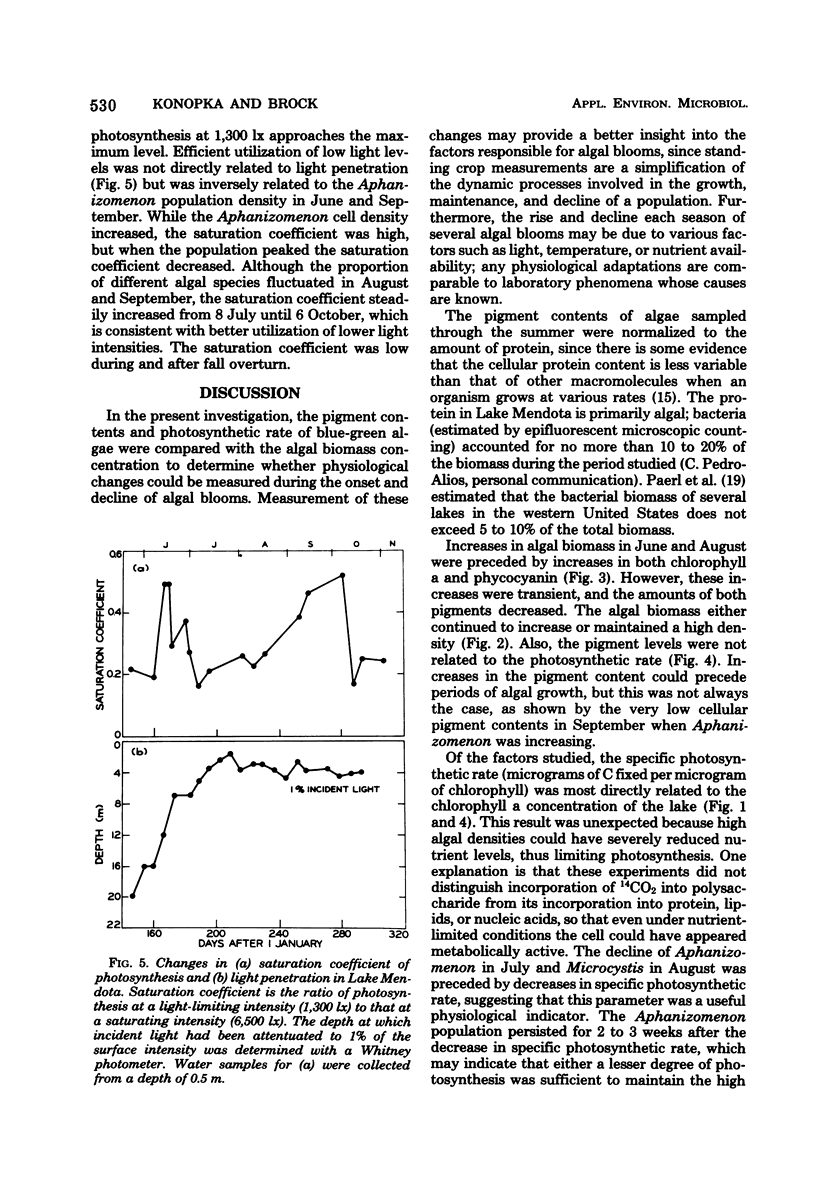
Blue-green algal blooms were present in Lake Mendota (Dane County, Wis.) from June to November 1976. Concentrations of total algal biomass and of particular algal species were monitored and compared with the pigment contents (chlorophyll a and phycocyanin) and photosynthetic rate of the algal populations. The specific photosynthetic rate (micrograms of C fixed per microgram of chlorophyll a per hour) was a good measure of the physiological state of the algae because this quantity increased just before each population increase and decreased before algal densities diminished. Since the quantity of light in the epilimnion which was available for photosynthesis by algal cells decreased in summer when the high algal densities attenuated incoming radiation, we investigated the possibility that the organisms would utilize lower light intensities more efficiently by increasing their pigment contents. Although some evidence of enhanced utilization of low light levels was found in the period from July to October, this result was not due to increasing chlorophyll and phycocyanin contents. There was a decrease in the phycocyanin content of the algae during this period, perhaps related to the availability of inorganic nitrogen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M. Photosynthetic membrane system in Anacystis nidulans. J Bacteriol. 1968 Sep;96(3):836–841. doi: 10.1128/jb.96.3.836-841.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Govindjee Transfer of the excitation energy in Anacystis nidulans grown to obtain different pigment ratios. Biophys J. 1966 Sep;6(5):611–619. doi: 10.1016/S0006-3495(66)86681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MYERS J., KRATZ W. A. Relation between pigment content and photosynthetic characteristics in a blue-green algae. J Gen Physiol. 1955 Sep 20;39(1):11–22. doi: 10.1085/jgp.39.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. T., Brock T. D. Adaptation by hot spring phototrophs to reduced light intensities. Arch Microbiol. 1977 May 13;113(1-2):111–120. doi: 10.1007/BF00428590. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


