Abstract
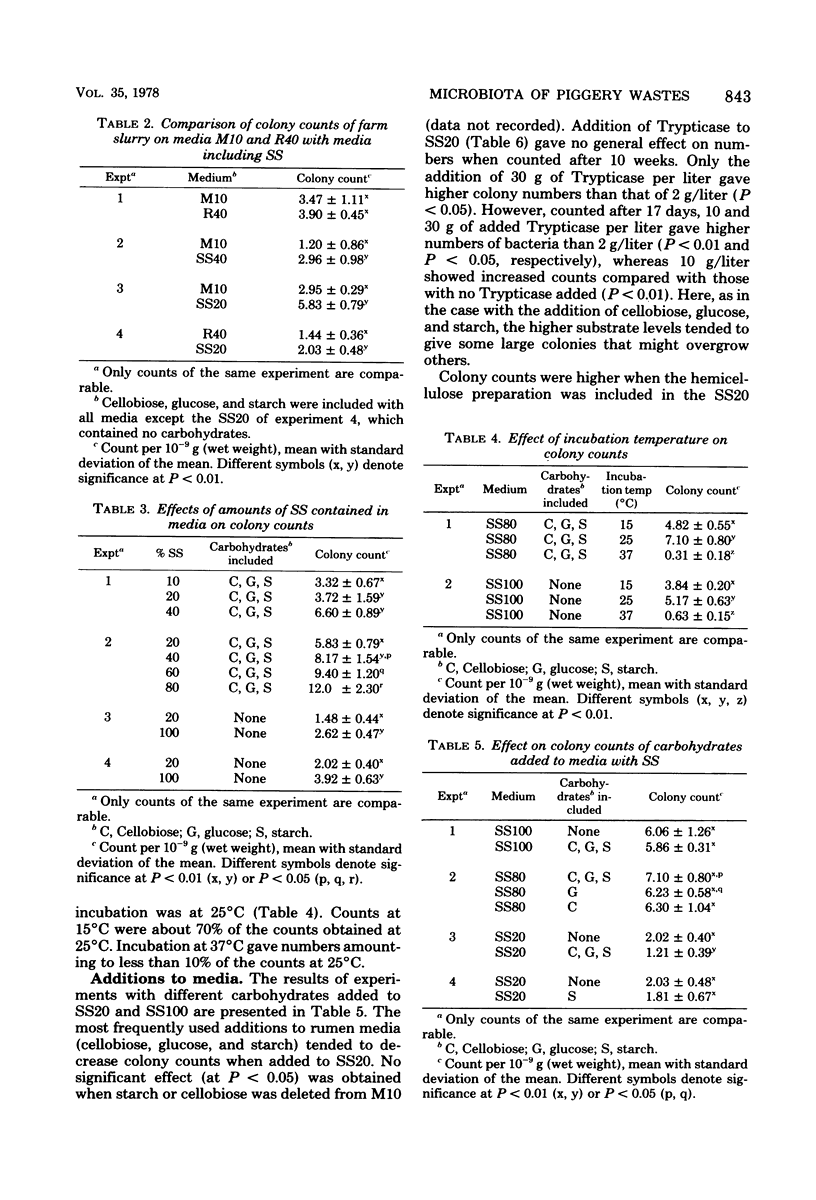
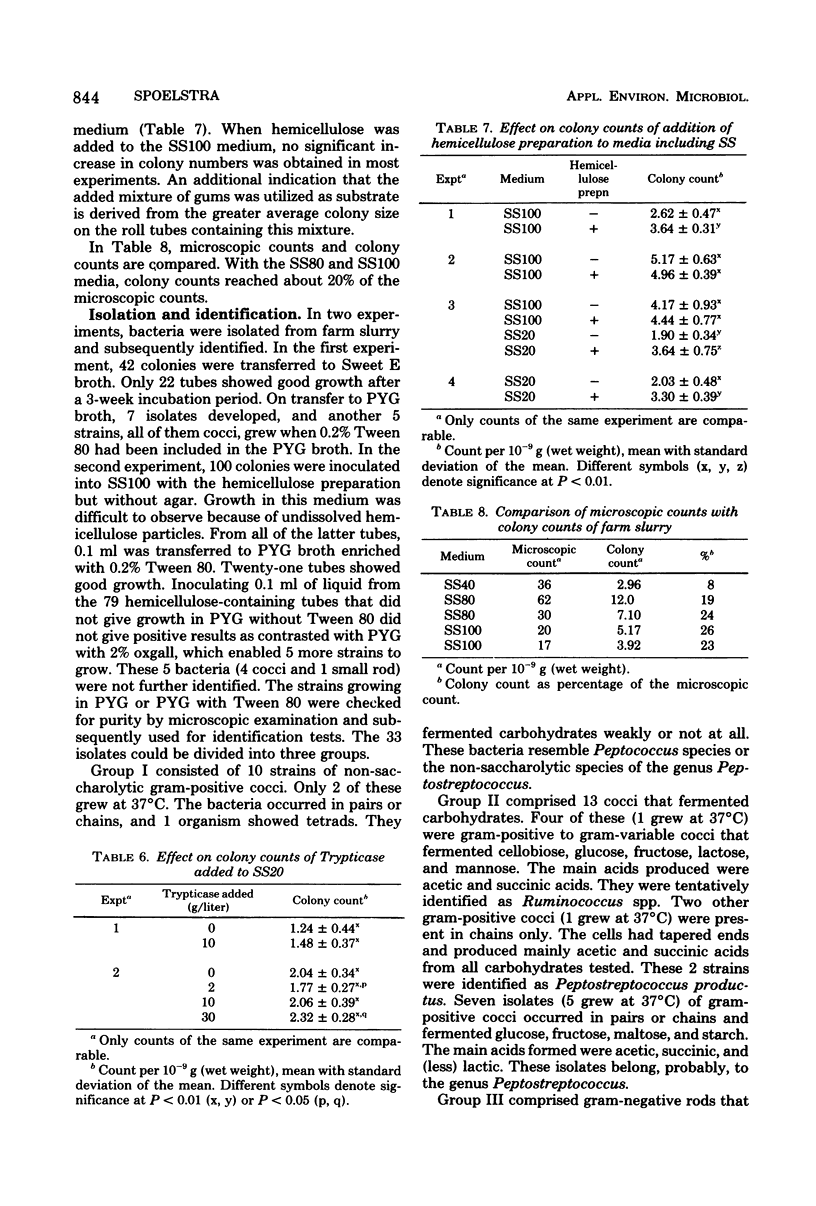
Media for enumeration of the microbiota of anaerobically stored piggery wastes were tested. Highest colony counts were obtained with 80 to 100% farm slurry supernatant included in the anaerobic roll tube media. Colony counts with these media numbered 2 X 10(9) to 12 X 10(9)/g (wet weight), which represents about 20% of the microscopic counts. Lower percentages of slurry supernatant in the media gave lower colony counts. Addition of glucose, cellobiose, and starch or of Trypticase to media with 20% slurry supernatant did not increase colony counts. Higher values were obtained when hemicellulose preparations were added to these media. Incubation at 25 degrees C gave the highest numbers. Incubation at 15 to 37 degrees C gave counts of about 70 and 10%, respectively, of those at 25 degrees C. Of the colonies picked for isolation, about 20% were obtained in pure culture. The isolates apparently belonged to the genera Peptococcus, Ruminococcus, Peptococcus, Ruminococcus, Pepostreptococcus, and Bacteroides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. D., Brock T. D. The temperature optimum of the intestinal flora of the rat. Can J Microbiol. 1968 Jun;14(6):699–704. doi: 10.1139/m68-116. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional features and ecology of predominant anaerobic bacteria of the intestinal tract. Am J Clin Nutr. 1974 Nov;27(11):1313–1319. doi: 10.1093/ajcn/27.11.1313. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. T., Hungate R. E. Effect of alfalfa fiber substrate on culture counts of rumen bacteria. Appl Environ Microbiol. 1976 Oct;32(4):649–652. doi: 10.1128/aem.32.4.649-652.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Hemicellulose degradation by rumen bacteria. Fed Proc. 1973 Jul;32(7):1819–1825. [PubMed] [Google Scholar]
- Grubb J. A., Dehority B. A. Variation in colony counts of total viable anaerobic rumen bacteria as influenced by media and cultural methods. Appl Environ Microbiol. 1976 Feb;31(2):262–267. doi: 10.1128/aem.31.2.262-267.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


