Abstract
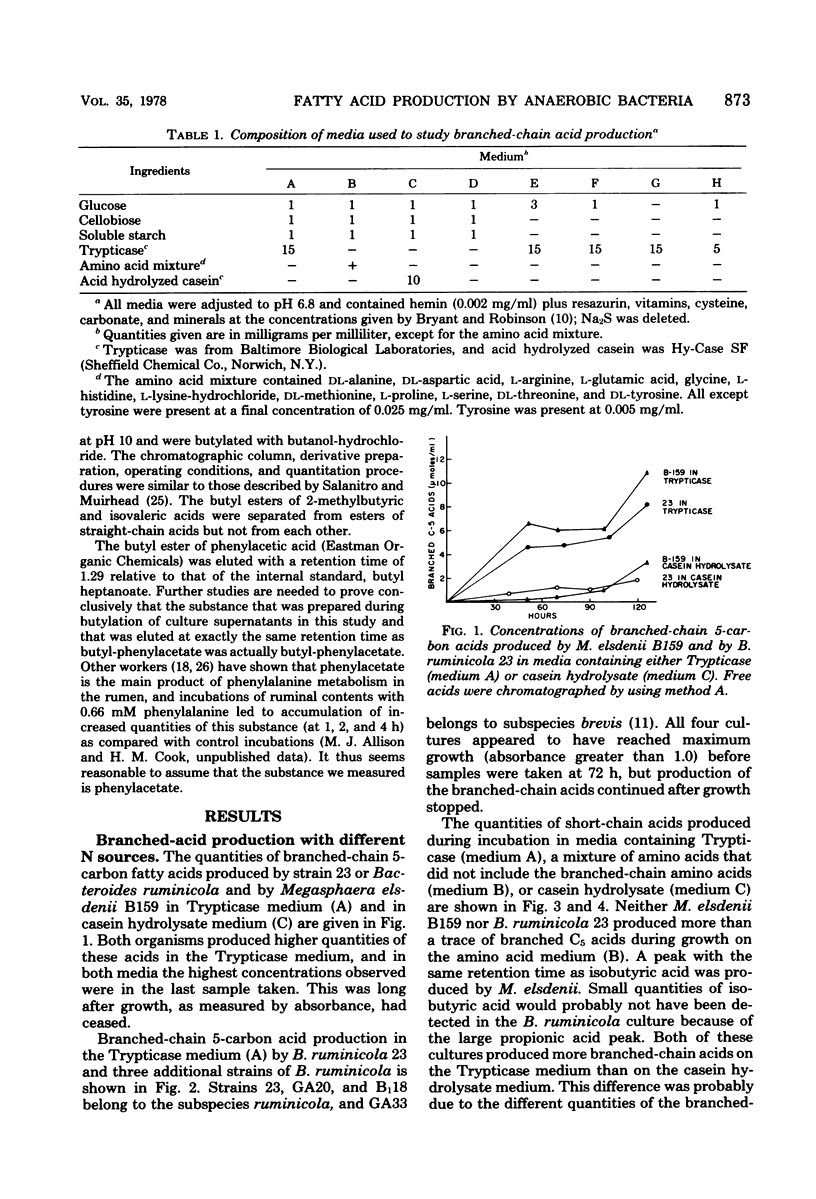
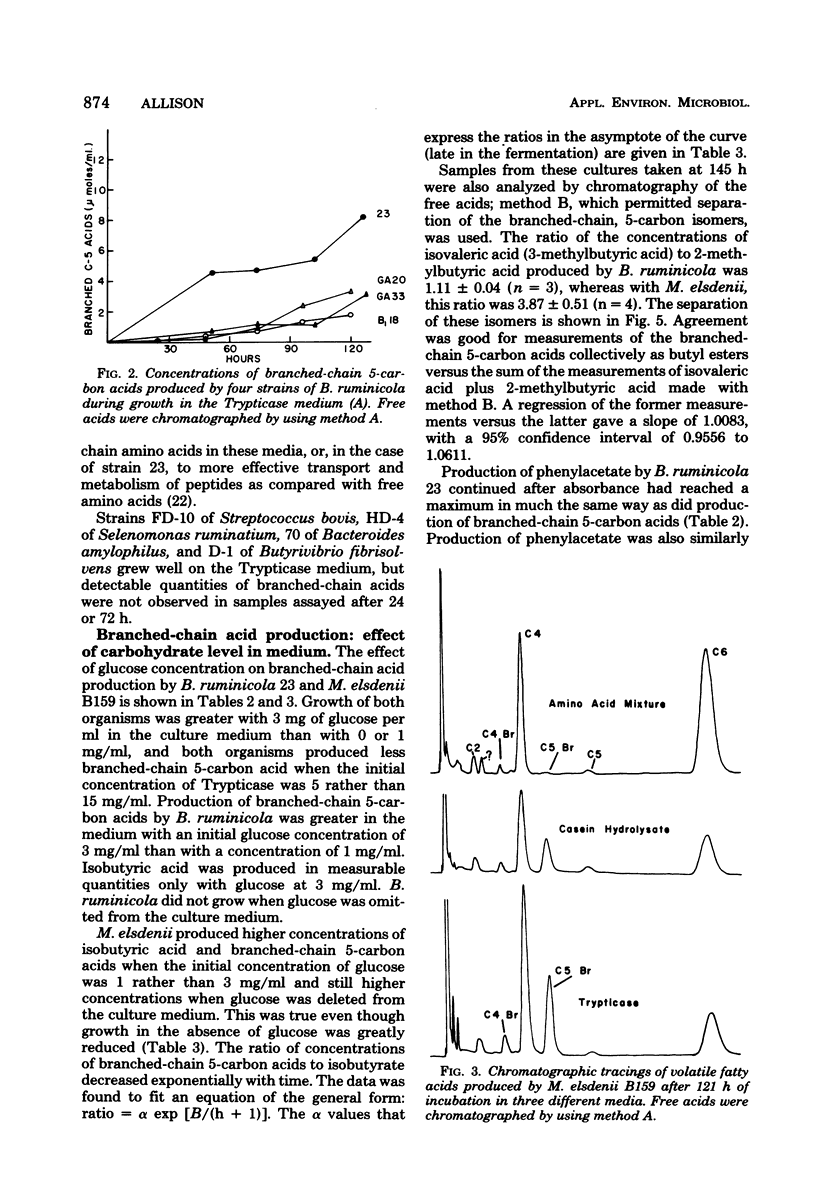
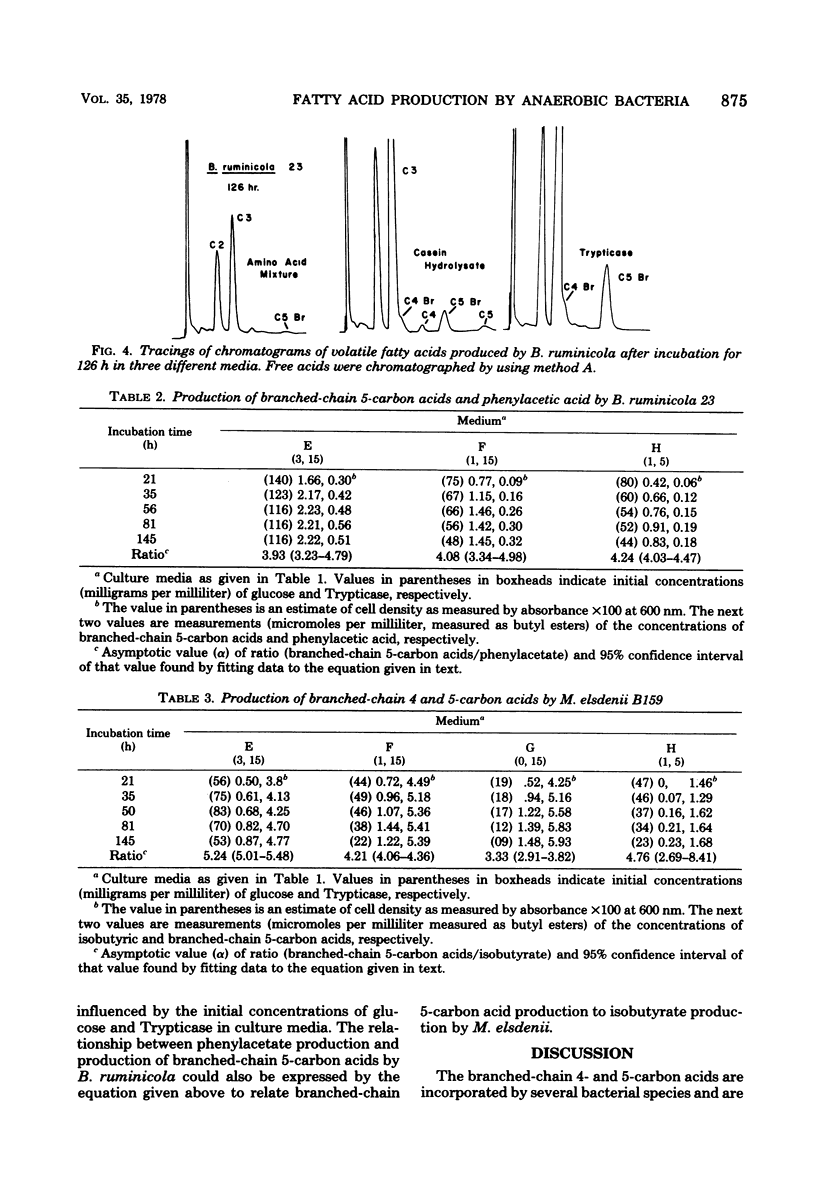
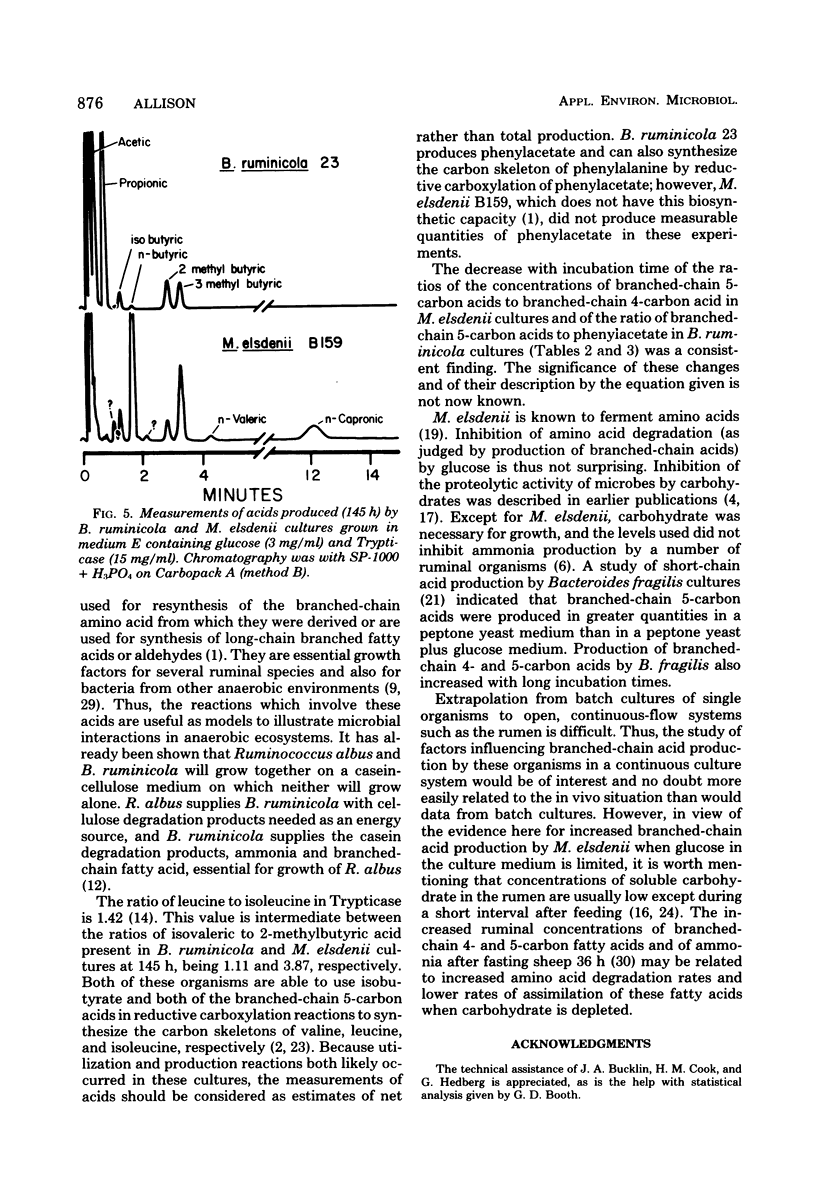
Net production of isobutyric acid, isovaleric acid, and 2-methylbutyric acid by cultures of Bacteroides ruminicola and Megasphaera elsdenii on media that contained Trypticase or casein hydrolysate continued (up to 5 days) after growth had ceased. Only trace quantities of these acids were produced in a medium that contained a mixture of amino acids that did not include the branched-chain amino acids. M. elsdenii produced increased quantities of the branched-chain fatty acids in a medium that contained Trypticase when glucose was reduced or eliminated from the culture medium. However, B. ruminicola produced increased quantities of branched-chain fatty acids and of phenylacetic acid from Trypticase when glucose was supplied at 3 mg/ml rather than at 1 mg/ml. Single strains of Streptococcus bovis, Selenomonas ruminantium, Bacteroides amylophilus, and Butyrivibrio fibrisolvens did not produce branched-chain fatty acids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F. Some observations on volatile fatty acids in the sheep's rumen. Biochem J. 1954 Jul;57(3):400–405. doi: 10.1042/bj0570400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J. Biosynthesis of amono acids by ruminal microorganisms. J Anim Sci. 1969 Nov;29(5):797–807. doi: 10.2527/jas1969.295797x. [DOI] [PubMed] [Google Scholar]
- Allison M. J., Bucklin J. A., Robinson I. M. Importance of the isovalerate carboxylation pathway of leucine biosynthesis in the rumen. Appl Microbiol. 1966 Sep;14(5):807–814. doi: 10.1128/am.14.5.807-814.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman N., Rettger L. F. The Influence of Carbohydrate on the Nitrogen Metabolism of Bacteria. J Bacteriol. 1918 Jul;3(4):389–402. doi: 10.1128/jb.3.4.389-402.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Bryant M. P., Doetsch R. N. A Study of Bacterial Species from the Rumen Which Produce Ammonia from Protein Hydrolyzate. Appl Microbiol. 1961 Mar;9(2):175–180. doi: 10.1128/am.9.2.175-180.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional features and ecology of predominant anaerobic bacteria of the intestinal tract. Am J Clin Nutr. 1974 Nov;27(11):1313–1319. doi: 10.1093/ajcn/27.11.1313. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEHORITY B. A., JOHNSON R. R., BENTLEY O. G., MOXON A. L. Studies on the metabolism of valine, proline, leucine and isoleucine by rumen microorganisms in vitro. Arch Biochem Biophys. 1958 Nov;78(1):15–27. doi: 10.1016/0003-9861(58)90310-2. [DOI] [PubMed] [Google Scholar]
- EL-SHAZLY K. Degradation of protein in the rumen of the sheep. I. Some volatile fatty acids, including branched-chain isomers, found in vivo. Biochem J. 1952 Aug;51(5):640–647. doi: 10.1042/bj0510640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-SHAZLY K. Degradation of protein in the rumen of the sheep. II. The action of rumen micro-organisms on amino acids. Biochem J. 1952 Aug;51(5):647–653. doi: 10.1042/bj0510647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACOSTE A. M., BLAIZOT J., RAYNAUD P. Catabolisme de la phénylalanine par les bactéries de la panse des ruminants. C R Hebd Seances Acad Sci. 1958 Feb 24;246(8):1280–1281. [PubMed] [Google Scholar]
- LEWIS D., ELSDEN S. R. The fermentation of L-threonine, L-serine, L-cysteine and acrylic acid by a gram-negative coccus. Biochem J. 1955 Aug;60(4):683–692. doi: 10.1042/bj0600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew J. W., Onderdonk A. B., Gorbach S. L. Effects of time and growth media on short-chain fatty acid production by Bacteroides fragilis. Appl Microbiol. 1975 Apr;29(4):472–475. doi: 10.1128/am.29.4.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman K. A., Lakshmanan S., Bryant M. P. Oligopeptide uptake by Bacteroides ruminicola. J Bacteriol. 1967 May;93(5):1499–1508. doi: 10.1128/jb.93.5.1499-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYAN R. K. CONCENTRATIONS OF GLUCOSE AND LOW-MOLECULAR-WEIGHT ACIDS IN THE RUMEN OF SHEEP CHANGED GRADUALLY FROM A HAY TO A HAY-PLUS-GRAIN DIET. Am J Vet Res. 1964 May;25:653–659. [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J. Isoleucine biosynthesis from 2-methylbutyric acid by anaerobic bacteria from the rumen. J Bacteriol. 1969 Mar;97(3):1220–1226. doi: 10.1128/jb.97.3.1220-1226.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOCRANSKY S. S., LOESCHE W. J., HUBERSAK C., MACDONALD J. B. DEPENDENCY OF TREPONEMA MICRODENTIUM ON OTHER ORAL ORGANISMS FOR ISOBUTYRATE, POLYAMINES, AND A CONTROLLED OXIDATION-REDUCTION POTENTIAL. J Bacteriol. 1964 Jul;88:200–209. doi: 10.1128/jb.88.1.200-209.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T. W., Ward P. F., Dawson R. M. The formation and metabolism of phenyl-substituted fatty acids in the ruminant. Biochem J. 1964 Jan;90(1):12–24. doi: 10.1042/bj0900012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenák I., Várady J., Boda K., Havassy I. Relationship between ammonia and volatile fatty acid levels in the rumen of fasting sheep. Physiol Bohemoslov. 1972;21(5):531–537. [PubMed] [Google Scholar]


