Abstract
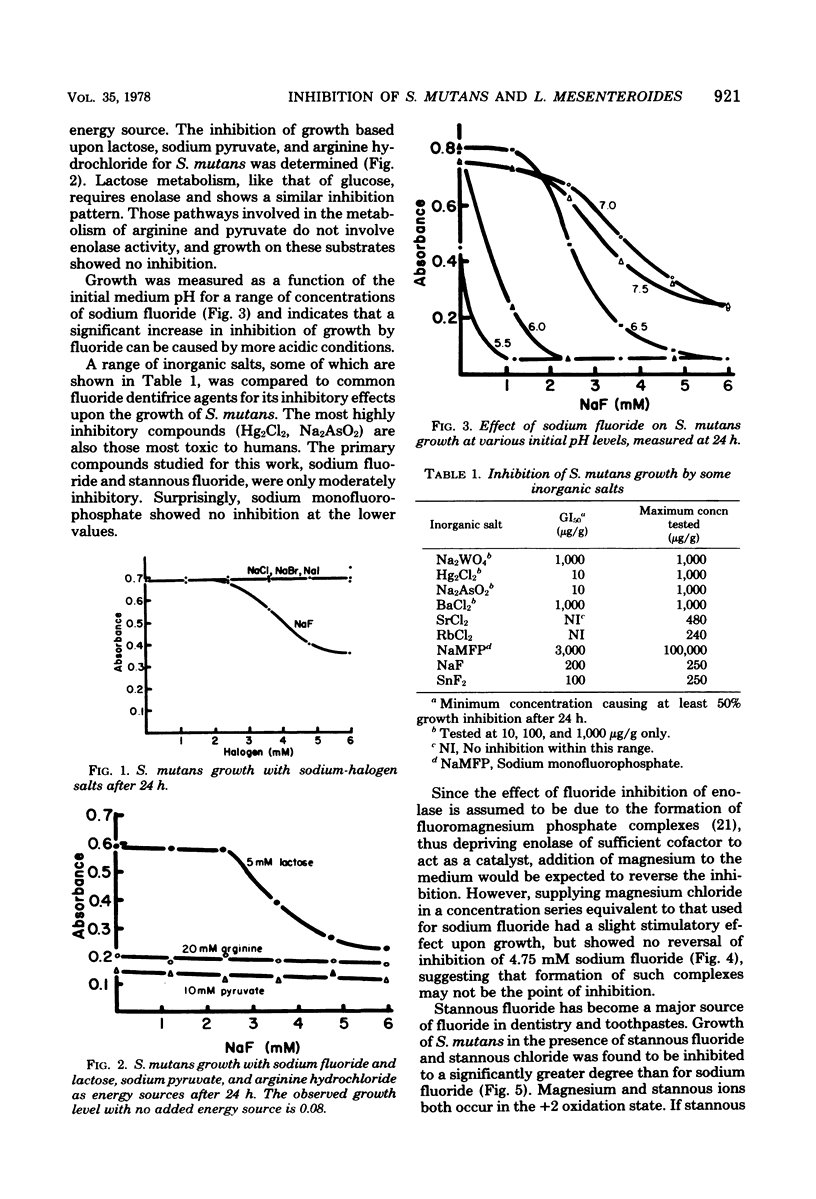
Sodium fluoride caused inhibition of growth rate and growth levels of Streptococcus mutans with glucose as the primary energy and carbon source. Stannous fluoride increased growth lag nad caused a much greater inhibition of growth rate than did sodium fluoride. Neither compound was found to be bactericidal when culture viability was measured after 6 days of incubation. Leuconostoc mesenteroides, which lacks a phosphotransferase system for sugar transport, showed less inhibition of growth rate with both inhibitors than did S. mutans, which possesses a phosphotransferase system. Metabolism of glucose or lactose which requires enolase activity shoed sodium fluoride inhibition, whereas metabolism of arginine or pyruvate does not involve enolase activity and showed no inhibition of growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWES C., JENKINS G. N., HARDWICK J. L., LEACH S. A. THE RELATION BETWEEN THE FLUORIDE CONCENTRATIONS IN THE DENTAL PLAQUE AND IN DRINKING WATER. Br Dent J. 1965 Aug 17;119:164–167. [PubMed] [Google Scholar]
- Horowitz H. S. Fluoride: research on clinical and public health applications. J Am Dent Assoc. 1973 Oct;87(5):1013–1018. doi: 10.14219/jada.archive.1973.0015. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- Kanapka J. A., Hamilton I. R. Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch Biochem Biophys. 1971 Sep;146(1):167–174. doi: 10.1016/s0003-9861(71)80053-x. [DOI] [PubMed] [Google Scholar]
- Keyes P. H. Research in dental caries. J Am Dent Assoc. 1968 Jun;76(6):1357–1373. doi: 10.14219/jada.archive.1968.0186. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Chemotherapy of dental plaque infections. Oral Sci Rev. 1976;9:65–107. [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Murray R. J., Mellberg J. R. Effect of topical acidulated phosphate fluoride on percentage of Streptococcus mutans and Streptococcus sanguis in plaque. II. Pooled occulusal and pooled approximal samples. Caries Res. 1975;9(2):139–155. doi: 10.1159/000260153. [DOI] [PubMed] [Google Scholar]
- Rölla G., Melsen B. Desorption of protein and bacteria from hydroxyapatite by fluoride and monofluorophosphate. Caries Res. 1975;9(1):66–73. doi: 10.1159/000260144. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Singer L., Jarvey B. A., Venkateswarlu P., Armstrong W. D. Fluoride in plaque. J Dent Res. 1970 Mar-Apr;49(2):455–455. doi: 10.1177/00220345700490024301. [DOI] [PubMed] [Google Scholar]
- Wang T., Himoe A. Kinetics of the rabbit muscle enolase-catalyzed dehydration of 2-phosphoglycerate. Fluoride and phosphate inhibition. J Biol Chem. 1974 Jun 25;249(12):3895–3902. [PubMed] [Google Scholar]


