Abstract
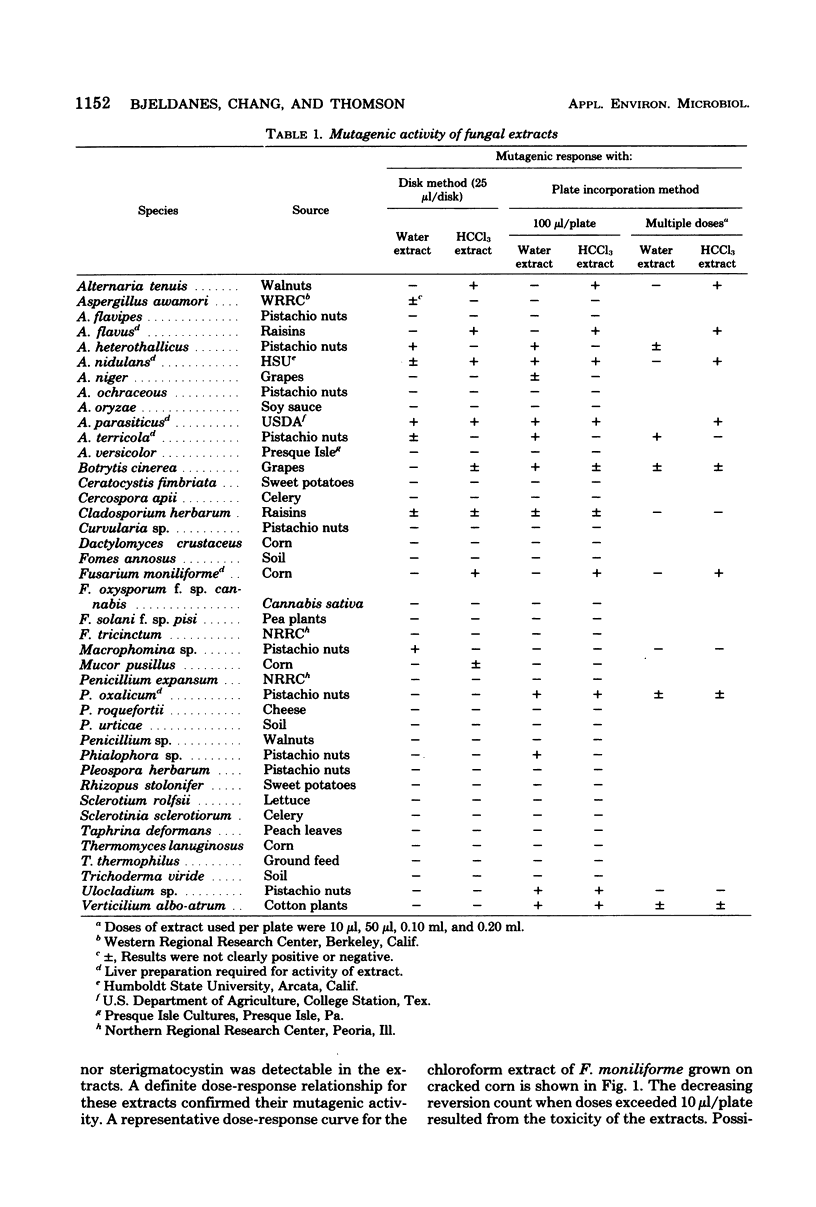
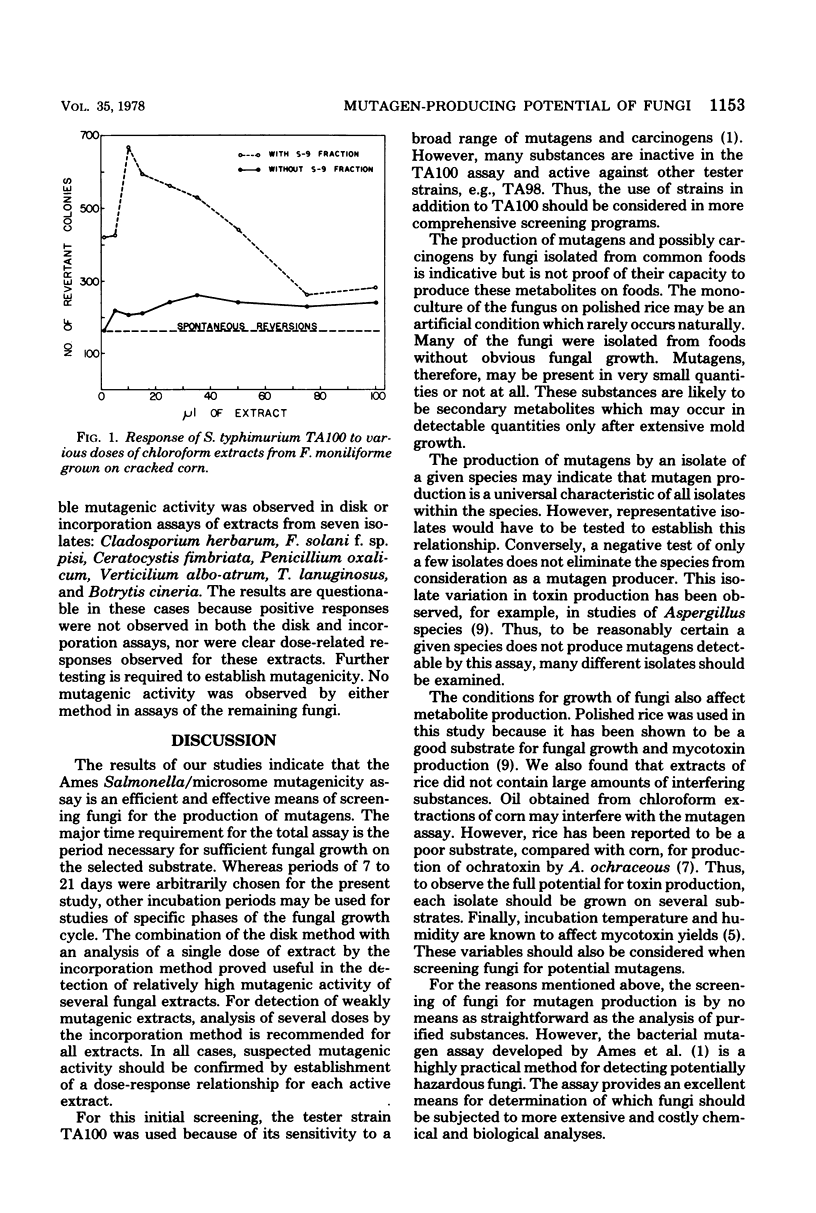
Forty-one fungal isolates (one isolate per species) representing common plant pathogens and food crop contaminants were grown on sterile, polished rice and assayed for mutagenic activity in the Salmonella typhimurium-microsome system. Initially, single doses of aqueous and chloroform extracts of the moldy rice were assayed against the TA100 tester strain by incorporating extracts into the growth medium and by applying small quantities on disks placed on the agar surface. Suspected activity was examined further by analysis of several doses in the plate incorporation assay. Extracts of two aflatoxin-producing isolates (Aspergillus flavus and A. parasiticus) showed pronounced mutagenic activity, as did extracts of five other isolates (A. heterothallicus, A. nidulans, A. terricola, Alternaria tenuis, and Fusarium moniliforme) which did not contain detectable aflatoxins. Seven additional isolates (Botrytis cineria, Ceratocystis fimbriata, Cladosporium herbarum, Fusarium solani f. sp. pisi, Penicillium oxalicum, Thermomyces lanuginosus, and Verticilium albo-atrum) revealed activity which was possibly mutagenic; i.e., mutagenic responses were not observed in both the disk and incorporation assays, and clear dose-related activity was not observed in the incorporation assay. Extracts of the remaining fungi were not mutagenic in the bacterial assay.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Barnes J. M., Austwick P. K., Carter R. L., Flynn F. V., Peristianis G. C., Aldridge W. N. Balkan (endemic) nephropathy and a toxin-producing strain of Penicillium verrucosum var cyclopium: An experimental model in rats. Lancet. 1977 Mar 26;1(8013):671–675. doi: 10.1016/S0140-6736(77)92115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank F., Chin O., Just G., Meranze D. R., Shimkin M. B., Wieder R. Carcinogens from fungi pathogenic for man. Cancer Res. 1968 Nov;28(11):2276–2281. [PubMed] [Google Scholar]
- Davis N. D., Wagener R. E., Morgan-Jones G., Diener U. L. Toxigenic thermophilic and thermotolerant fungi. Appl Microbiol. 1975 Apr;29(4):455–457. doi: 10.1128/am.29.4.455-457.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesseltine C. W., Vandegraft E. E., Fennell D. I., Smith M. L., Shotwell O. L. Aspergilli as ochratoxin producers. Mycologia. 1972 May-Jun;64(3):539–550. [PubMed] [Google Scholar]
- Miller J. A., Miller E. C. Carcinogens occurring naturally in foods. Fed Proc. 1976 May 1;35(6):1316–1321. [PubMed] [Google Scholar]
- Mislivec P. B., Dieter C. T., Bruce V. R. Mycotoxin-producing potential of mold flora of dried beans. Appl Microbiol. 1975 Apr;29(4):522–526. doi: 10.1128/am.29.4.522-526.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodricks J. V. The occurrence and control of mycotoxins and mycotoxicoses. Food Nutr (Roma) 1976;2(1):9–14. [PubMed] [Google Scholar]
- Romer T. R. Screening method for the detection of aflatoxins in mixed feeds and other agricultural commodities with subsequent confirmation and quantitative measurement of aflatoxins in positive samples. J Assoc Off Anal Chem. 1975 May;58(3):500–506. [PubMed] [Google Scholar]


