Abstract
In 2004, canine influenza virus (CIV) was identified as a respiratory pathogen of dogs for the first time and is closely related to H3N8 equine influenza virus (EIV). We generated a recombinant vectored vaccine that expresses H3 of a recent isolate of EIV using equine herpesvirus type 1 (EHV-1) as the delivery vehicle. This EHV-1 vectored vaccine exhibited robust and stable EIV H3 expression and induced a strong influenza virus-specific response in both mice and dogs upon intranasal or subcutaneous administration. Furthermore, upon challenge with the recent CIV isolate A/canine/PA/10915-07, protection of vaccinated dogs could be demonstrated by a significant reduction in clinical sings, and, more importantly, by a significant reduction in virus shedding. We concluded that the EHV-1/H3 recombinant vector can be a valuable alternative for protection of dogs against clinical disease induced by CIV and can significantly reduce spread.
Keywords: EHV-1, canine influenza, H3
Introduction
Interspecies transmission, through genetic reassortment or direct transfer, accounts for most of the evolutionary success of influenza A viruses [1]. Canine influenza virus (CIV) emerged recently as the result of a direct transmission of equine influenza virus (EIV) H3N8 subtype into dogs [2]. EIV infection is the leading cause of respiratory disease in the horse, and two major virus subtypes, H7N7 and H3N8, have been isolated from affected equids, although the H7N7 has not been isolated for more than two decades [3]. The H3N8 subtype, however, has not been successfully controlled by current vaccination strategies, and continues to pose a serious threat to horse welfare. In addition, the apparent ability of the H3N8 horse virus to jump species, the threat of clinical disease in other animals such as the dog is imminent [3, 4].
CIV was first recognized in racing greyhounds in the US in Florida in 2004 and since has rapidly spread among racing and pet dogs throughout several other states in the US [2, 5]. The clinical signs associated with CIV infection range from subclinical to acute respiratory disease characterized by pyrexia and cough, which generally lasts around 10–14 days. Most affected animals will recover from infection, although death has been reported as a result of hemorrhages in the respiratory tract [2]. To date, no effective vaccine against CIV has been licenced, and a specific treatment for the disease is unavailable.
Equine herpesvirus type 1 (EHV-1) is an Alphaherpesvirus of the genus Varicellovirus and predominates in the horse population as a respiratory pathogen, occasionally causing abortions and neurological disease [6, 7]. The EHV-1 modified-live virus vaccine strain RacH is commonly used to vaccinate horses against EHV-1 in Europe and in the US. RacH is innocuous in mice and horses and its attenuation could be attributed to a deletion of both copies of gene 67 (IR6), which arose spontaneously during its 256 passages on primary swine kidney cells. Other genomic alterations such as truncation of the glycoprotein B also contribute to its attenuation in a variety of species [8–10]. RacH-based vaccine vectors stably and efficiently deliver immunogenic proteins, induce both humoral and cellular immune responses, and they may also protect vaccinated animals from heterologous challenge [11–13]. EHV-1 RacH has a broad host range in cultured cells and infects both dividing and non-dividing cells from different hosts including canine cells [14].
In the present study, we developed a RacH-based vaccine that expresses H3 derived from the EIV subtype H3N8 and that was named rH_EIV. We showed that rH_EIV retains expression of H3 over several passages without impairing its ability to replicate in cultured cells. Furthermore, rH_EIV induces robust immune responses in mice and dogs, and partially protects dogs against challenge with virulent CIV by significantly reducing clinical signs and virus shedding.
Materials & Methods
Cells and viruses
Rabbit kidney (RK13) and Madin–Darby bovine kidney (MDBK) cells were maintained in Earle's minimum essential medium (EMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 0.1mg/ml streptomycin (1% Pen/Strep; Mediatech, Inc.). Parental virus HΔgp2 (EHV-1 strain RacH in which gene 71 was replaced with mini-F sequences) [15] and rH_EIV (recombinant EHV-1 expressing H3) were propagated in RK13 cells. The influenza virus used for challenge, A/canine/PA/10915-07 (H3N8) (Dubovi, unpublished observation), was isolated from an outbreak of canine influenza in Pittsburgh, PA, in 2007 and was propagated in chicken eggs.
Generation of vaccine virus
The H3 gene of equine influenza (EIV) strain A/equine/OH/03 (H3N8), was commercially synthesized after codon-optimization (sequence available upon request, Geneart, Inc) and cloned into shuttle plasmid pEP_goi- in [13, 16]. Two-step Red recombination [16] was used to insert EIV H3 into the infectious bacterial artificial chromosome (BAC) clone of the EHV-1 vaccine strain RacH (pRacH1). This recombinant pH_EIV and parental pRacH1 DNA was isolated, digested, and analyzed by 0.8% agarose gel electrophoresis after visualization of DNA fragments with ethidium bromide (EtBr) as described previously [13]. Recombinant and parental viruses were reconstituted after transfection of 2µg of pH_EIV or pRacH1 DNA into RK13 cells by calcium phosphate precipitation. Two days post-transfection, virus-containing supernatants were clarified from cellular debris, viruses were harvested, titered by plaque assay as described below, and stored frozen at −80°C [13, 15].
Indirect immunofluorescence (IF) analysis
For IF analyses, RK13 cells were infected at a multiplicity of infection (MOI) of 0.0001 with rH_EIV. One hour post-infection (hpi), medium was removed and infected cells were overlaid with 0.25% methylcellulose in EMEM-10% FBS. Two days post-infection (dpi), cells were fixed with 90% ice-cold acetone for 10 minutes at −20°C. After re-hydration with PBS, cells were blocked using PBS-0.5% bovine serum albumin (BSA) for 30 minutes at room temperature (RT). Cells were incubated for 30min at RT with monoclonal antibody (mAb) 3E5.2 specifc for EIV H3 at a1:5 dilution (kindly provided by Dr. Judy Appleton, Cornell University) or mAb F7 directed against EHV-1 gM (1:50 dilution) [15] for 30 min at RT. After extensive washing with phosphate-buffered saline (2.5mMol NaH2PO4, 7.5 mMol Na2HPO4, 145mmol NaCl, pH 7.2) (PBS), a 1:500 dilution in PBS of Alexa Fluor488-conjugated goat anti-mouse immunoglobulin (Ig) G (Molecular Probes) was added for 30 minutes at RT. Plaques were inspected and photographed after thorough washing using an inverted fluorescent microscope (Zeiss Axiovert 25 and Axiocam). Viruses were continuously passaged using an MOI of 0.01 until all cells exhibited cythopatic effect (CPE), and expression of H3 was verified by IF every three passages until passage level 10.
In vitro replication assays
To determine replication of the recombinant virus, single-step replication kinetics and plaque areas were determined. Plaque areas on RK13 cells were measured after infection of cells seeded in a 6-well plate at an MOI of 0.0001 and overlay with EMEM-10% FBS containing 0.25% methylcellulose at 1 hpi. At 3 dpi, plaques were analyzed by IF using mAb F7; 50 plaques were photographed, and average plaque areas were determined using the ImageJ software (http://rsb.info.nih.gov/ij/). Values were compared to HΔgp2 plaque diameters, which were set to 100%. Average percentages of plaque areas were determined from at least three independent experiments. Single-step growth kinetics were determined after infection of 1X105 RK13 cells at an MOI of 3. Virus was allowed to attach for 1 h at 4°C, followed by a penetration period of 1.5 h at 37°C. At 0, 8, 16, 20 and 24 h after infection, supernatants and cells were harvested separately, and cell-associated and extracellular viral titers were determined by plating onto RK13 cells. At 3 dpi, cells were fixed with 10% formalin in PBS for a plaque assay, stained with 0.3% crystal violet, and plaques were counted. Single-step growth curves were determined in three independent experiments.
Mouse experiment
All animal experiments were performed in accordance with the United States Animal Welfare Act, under the supervision of the Cornell Institutional Animal Care and Use Committee. Three-week-old female BALB/c mice (Harlan) were randomly allocated into four groups of three mice each and inoculated intranasally (IN) three times in 3-week intervals. Three groups were inoculated with three different doses of rH_EIV (group 1: 1X103 PFU; group 2: 1X104 PFU; group 3: 1X105 PFU), while group 4 served as a negative control and received 1X105 PFU of HΔgp2. All mice were bled for serological testing on days 40 and 56 following the second and third inoculation. Serum was collected by centrifugation and haemagglutination inhibition (HI) assays were performed (see below).
Dog experiment
Eight purpose-bred intact beagle bitches (Marshall Farms), approximately 8 weeks of age, were placed in group housing for the purpose of blood collection and vaccination prior to challenge infection. The dogs were not segregated based on group affiliation. Individual dogs were identified by ear tattoos. None of the dogs had detectable antibodies to CIV, as determined by the HI assay, prior to vaccination.
Vaccination and challenge
The dogs were randomized into two groups of four and the allocation into groups of individual dogs remained unknown to the examiners after that time for the duration of the experiment and data evaluation. Dogs were inoculated subcutaneously (SC) and group 1 received 2.4 × 106 PFU rH_EIV while group 2 received virus resuspension buffer (negative control). The dogs received a booster vaccination of 4.1 × 106 PFU of rH_EIV or resuspension buffer 4 weeks later. All dogs were challenged three weeks after booster vaccination with 1×106 PFU A/canine/PA/10915-07 using 2 ml of virus-containing allantoic fluid, which was placed in a custom-engineered nebulizer and was administered with flow-through oxygen to each individual dog for approximately 10 minutes.
Clinical observations
Physical examinations were performed 2 days prior to challenge, on the day of challenge (day 0) and from days 1 to 8, and 10 and 15 post challenge. Observation of the activity level, demeanor, heart rate, respiratory rate, rectal temperature, and overall general appearance were performed. A fever was defined as a rectal temperature above 39.5°C and scores for individual clinical signs were added to determine an overall clinical score. The demeanor was scored as a 0 or 1 distinguishing between BAR (bright, alert and responsive, 0) or not (1), The absence (0) or presence (1) of fever, the absence (0) or presence (1) of cough, the absence (0) or presence (1) of nasal discharge, and the absence (0) or presence (1) of ocular signs to include conjunctivitis and/or discharge.
Specimen collection
Blood was drawn from the vena cephalica on days −56 (initial vaccination), −35, −21 (booster vaccination), −14, and −7 before challenge. In addition, blood was drawn on days 0, 7, and 15 post challenge. Nasal swabs using one 6 cm non-sterile foam-tipped swab (Fisher) and one TX754B Mini Alpha swab (ITW Texwipe) were taken from each dog at the same time points as described above (Figure 4). The swabs were placed immediately in 1 ml of transport media (PBS supplemented with 10% glycerol, 4% penicillin/streptomycin/amphotericin B, 10% FBS, and 0.05% enrofloxacin (Baytril®) on ice.
Figure 4. Immunization of dogs with rH_EIV induces anti-H3 specific antibodies.
Two groups of four dogs were vaccinated s.c. twice in a 27 day interval with 2.4 × 106 PFU and 4.1×106 PFU of rH_EIV at day 0 and 28 respectively, or mock-vaccinated with resuspension buffer. (A) The relative percentage of serum inhibition was determined by an inhibition ELISA. Samples under 30% relative inhibition were considered negative, between 30 and 50% questionable, and samples above 50% inhibition were considered positive. The serum sample of one dog was not analysed at time points 14 and 35 days after first immunization. Asterisks indicate statistically significant differences. (B) Serum neutralizing antibodies were determined by a standard haemagglutinin inhibition (HI) assay in sera of vaccinated and mock-vaccinated dogs at the indicated time points post-vaccination.
Antibody tests
All sera were tested by use of standard microtiter HI and an inhibition enzyme-linked immunosorbent (ELISA) assay. The HI assay was performed exactly as described before [17]. For the inhibition ELISA, wells of 96-well microtiter plates (Nunc) were coated with 10 HA units/ml of allantoic fluid, obtained from eggs infected with the influenza strain H3N8-PR-8 (a gift from Dr. R. Donis, CDC, Atlanta, GA) in coating buffer (15mmol Na2CO3, 35mmol NaHCO3, pH 9.6) and incubated overnight at 4°C. Wells were blocked with 3% skim milk powder in PBS for 2h at RT. After washing with PBS containing 0.05% (v/v) Tween 20, a dilution series of dog serum was added to the wells and incubated for 2h at 37°C, followed by the mAb 11B4.6.7.2.1 specific for H3 (provided by Dr. Judy Appleton, Cornell University), which was diluted 1/2000 in 0.3% skim milk powder in PBS (1h at 37°C). Finally, peroxidase-conjugated anti mouse immunoglobulin G (diluted 1/5000 in 0.3% skim milk powder in PBS; Jackson Laboratories, Ben Harbor, ME) was added to the wells for 1h at 37°C. The substrate used was BD OptEIA reagent A and B (BD Biosciences, San Jose, CA) and the reactions were stopped with 1.8M H2SO4, after which the absorbance was determined at 450 nm in a microplate reader (Bio-Tek, Richmond, VA).
CIV shedding
Shedding of canine influenza virus was evaluated by virus isolation in eggs (qualitative assay) and in MDCK cells (quantitative assay), and by RT-PCR. For the egg isolations, 10 day old SPF embryonated eggs (2 per sample) were inoculated with 0.1 ml of nasal swab extract. Eggs were incubated for 4 days followed by a haemagglutination test for the presence of influenza virus. Negative eggs were passaged one more time before defining as negative. For the MDCK assay, serial 10-fold dilutions of nasal swab extract were added to microtiter plates (8 wells/dilution). Plates were incubated for 4 days and virus positive wells were detected by immunohistochemistry [18]. For RT-PCR reactions, RNA was extracted using a commercial kit (Ambion MagMAX 96 Viral RNA Isolation Kit) according to the manufacturer’s instructions with minor modifications, using the Kingfisher 96 Automated Extraction system (Thermo). Briefly, 20 µl of Bead Mix (10 µl of RNA Binding Beads to 10 µl of Lysis/Binding Enhancer), 130 µl of Viral Lysis/Binding Buffer (1 µl Carrier RNA to 65 µl lysis/binding concentrate and 65 µl 100% isopropanol), and 60 µl of sample was added to each well in a labeled 96-well plate. Samples were washed twice with 100 µl of wash solution I and II respectively, and RNA was eluted in 100 µl of RNase-free water. Real-time RT-PCR for the matrix gene of influenza A viruses was performed essentially as described previously [19]. The commercial one-step-RT PCR kit, Superscript III RT-PCR kit (Invitrogen), was used to amplify a highly conserved sequence of the influenza A virus matrix gene with primers AIM+25 (5’-AGATGAGTCTTCTAACCGAGGTCG-3’) and AIM-124 (5’-TGCAAAAACATCTTCAAGTCTCTG-3’), and probe M+36 (FAM-5’-TCAGGCCCCCTCAAAGCCGA-3’-BHQ1). A 25-µl reaction mixture included 12.5 µl of 2 X reaction buffer, 400 µM of each primer, 120 µM probe, 0.5 µl of RT-Taq enzyme mix, 0.5 µl of ROX (Invitrogen), 8 µl of RNA template, and RNase-free water. The RT-PCR conditions for the ABI 7000 Sequence Detection System were as follows: 1 cycle of RT for 30 min at 50°C, 1 cycle of DNA polymerase activation at 95°C for 2 min, followed by 45 cycles of 94°C for15 s, 60°C for 60 s.
Statistical analyses
Statistical analyses were performed using the JMP software package (version 6.0.3). Student’s t-test was used to test for differences in plaque areas and viral in vitro kinetics on individual days. Temperatures, clinical scores and virus shedding data were compared using the non-parametric Kruskal Wallis Wallis Rank Sum test without large-sample approximation. No adjustment for multiple comparisons was made since the total number of comparisons made was low and a comparably conservative level of significance was chosen. HI titers and ELISA data were analyzed using multivariate analysis of variance, treating observations on individual days on the same subject as multivariate responses (MANOVA).
Results
Generation and in vitro characterization of recombinant EHV-1 expressing H3 (rH_EIV)
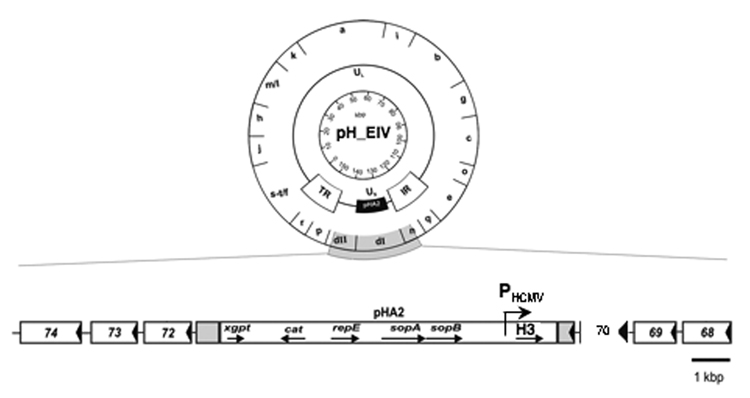
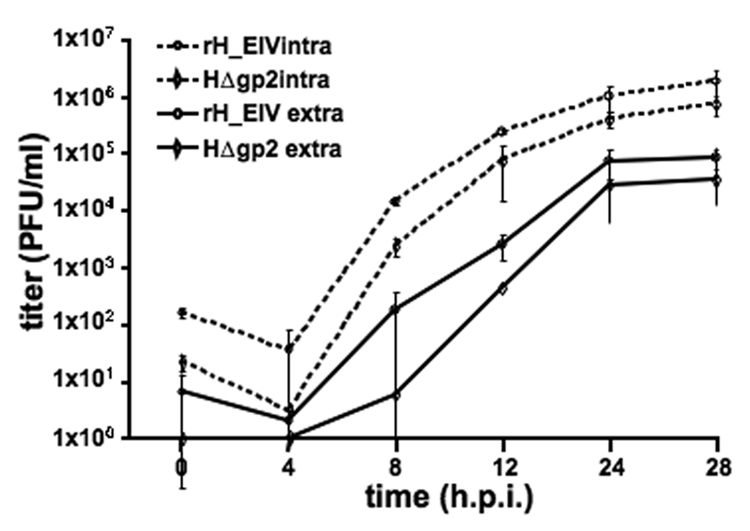
The H3 sequence from a recently isolated EIV strain, A/equine/OH/03, was codon-optimized for optimal and high-level expression in mammalian cells (Geneart, Inc) and synthesized. The sequence under the HCMV IE promotor was inserted into the EHV-1 RacH genome using the infectious pRacH1 clone and two-step en passant mutagenesis (Figure 1). Viruses were reconstituted from cloned DNA by transfection into RK13 cells and stable and high-level expression of H3 by the virus, rH_EIV, was demonstrated by indirect immunofluorescence (IF). As expected, H3 could only be detected in rH_EIV-infected cells but not in parental HΔgp2-infected cells, and a robust and stable expression of H3 was detected in rH_EIV-infected cells even after 10 continuous passages in RK13 cells of rH_EIV when passaging was stopped (data not shown). rH_EIV exhibited intracellular and extracellular growth rates that were comparable to those of parental HΔgp2 virus, and an approximately 2- to 3-fold increase in virus titers was observed at some time points p.i. (Figure 2). Plaques formed by the recombinant virus were slightly but not significantly smaller than those formed by the parental HΔgp2 virus (data not shown).
Figure 1. Generation of recombinant EHV-1 virus expressing H3 (rH_EIV).
Schematic representation of the construction of the rH_EIV vaccine vector based on pRacH. A detailed organization of the unique short region shows the insertion of the H3 gene under the HCMV promotor in the mini-F sequence (instead of gene 71 encoding gp2) of pRacH.
Figure 2. In vitro growth kinetics of rH_EIV.
RK13 cells were infected at an m.o.i. of 3 with rH_EIV or the parental HΔgp2 virus. Supernatant (extracellular) and cellular (cell-associated) fractions were collected at the indicated time points and virus titers were determined by standard plaque assay. The results are expressed as the mean ± STDEV of three independent experiments.
Serological responses induced in rH_EIV-immunized mice
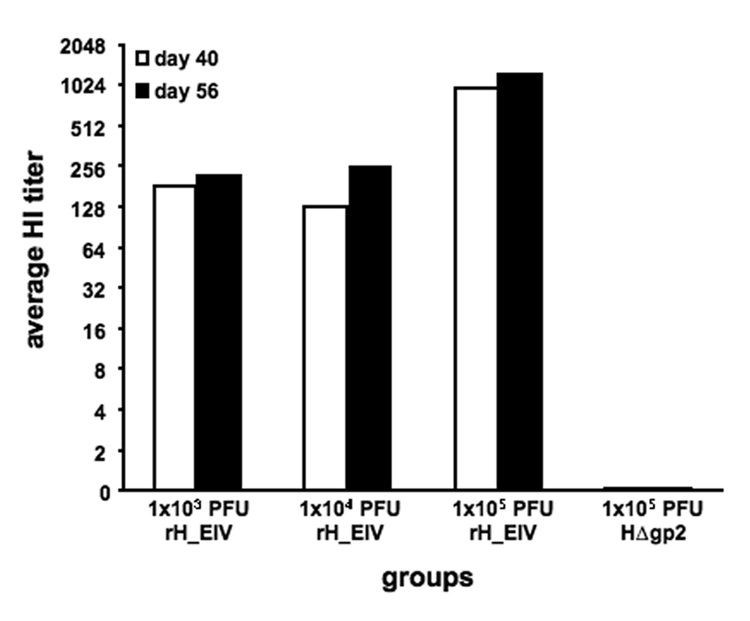
To determine if immunization of mice with rH_EIV induced humoral immune responses against H3N8 influenza virus, four groups of mice were inoculated intranasally (i.n.) three times at 3- week intervals. Sera collected at days 40 and 56 after the initial vaccination contained anti-H3 antibodies as measured by HI and was clearly dose-dependent, with the highest antibody levels at day 56 in animals immunized with 1×105 PFU (Figure 3). As expected, no HI titers were detected upon immunization with parental HΔgp2 virus (Figure 3). Lower, but detectable titers of H3-specific HI antibodies were also detected in the groups that received 10- or 100-fold less virus, even after two administrations. It is also noteworthy that three weeks after the second immunization, high levels of H3 antibodies were already present in mice, suggesting a strong humoral immune response to the H3 antigen after only two vaccinations (Figure 3).
Figure 3. Immunization of mice with rH_EIV induces anti-H3 specific antibodies.
Four groups of three mice were vaccinated i.n. three times in a 27 day interval. Group 1 received 1 × 103 PFU, group 2 1×104 PFU and group 3 1×105 PFU of rH_EIV. Group 4 received 1×105 PFU parental HΔgp2 virus. Mice were bled at days 40 and 56 post vaccination and serum neutralizing antibodies were determined by a standard haemagglutinin inhibition (HI) assay.
Serological responses induced in rH_EIV-immunized dogs
To determine if the immune responses observed in mice resembled those in our target species, an immunization study was performed in dogs. Four beagle dogs were inoculated twice in a 4-week interval by subcutaneous injection (s.c.) with rH_EIV, while four beagle dogs were mock-inoculated. Serum samples were tested by HI and also in a competition ELISA. The ELISA revealed presence of antibodies against H3 as early as 2 weeks after the first rH_EIV inoculation, whereas no H3 antibody levels above the cut-off value were detected in un- or mock-vaccinated dogs (Figure 4A). In addition, it was observed that the rH_EIV inoculated group maintained a steady level of antibodies up to 2 weeks after booster vaccination (Figure 4A). Similarly, the presence of functional antibodies against H3, as determined by HI assays, was found in vaccinated dogs. However, as compared to the newly developed ELISA test, such antibodies were only detected one week after booster vaccination (Figure 4B). In line with the results of the ELISA, H3-specific HI antibodies were not detected in mock-vaccinated animals (Figure 4B). From the results we concluded that the rH_EIV vectored vaccine was able to induce an early and robust immune response in the target species, the dog.
Immunization of dogs with rH_EIV results in partial protection upon experimental challenge with CIV
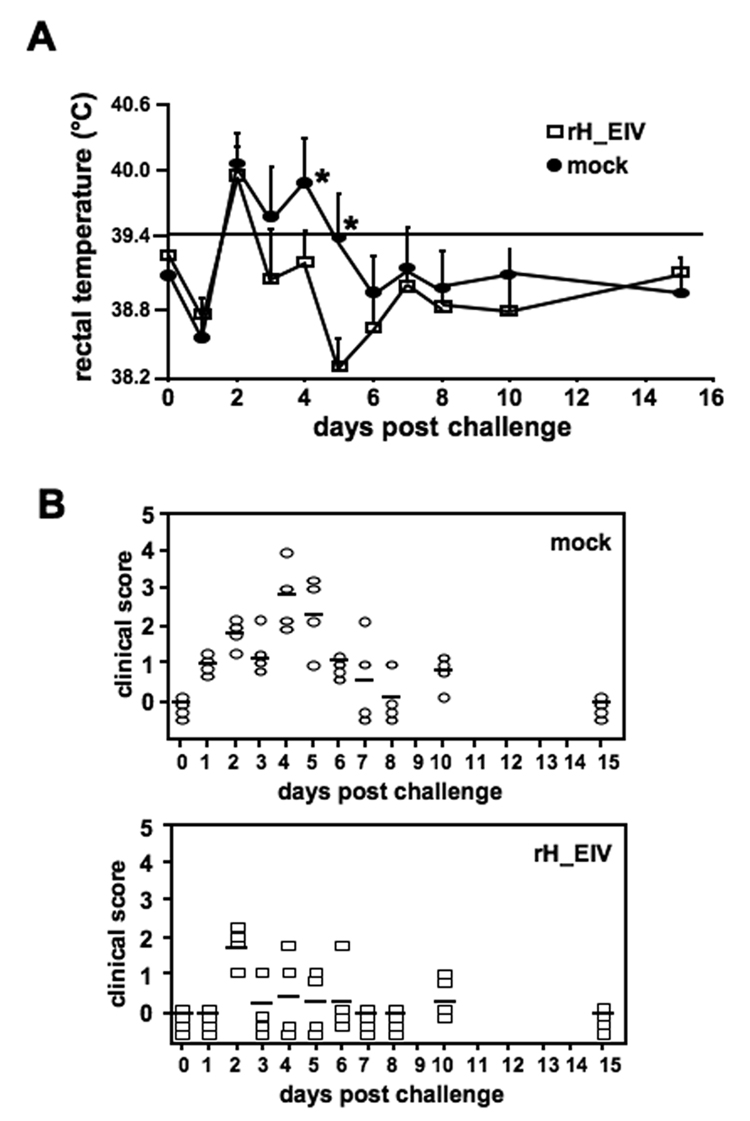
Two weeks after booster vaccination, all dogs were challenged with 1×106 PFU of CIV. Following challenge infection, all individuals in both groups experienced an initial decline in rectal temperature, which was concomitant with a reduction in activity, likely associated with the stress of challenge on the previous day (Figure 5A). However, the clinical observations indicated that individuals in the vaccinated group were more bright and alert than those in the mock-vaccinated control group (Figure 5B). On the second day following challenge, all dogs presented with a fever (Figure 5A), which had already resolved one day later (day 3) in the vaccinated group, whereas the unvaccinated group only reached average rectal temperatures within normal limits by day 6. Statistical testing revealed that vaccination significantly reduced fever (Figure 5A, p<0.01). When evaluating the clinical signs, it became apparent that both groups experienced some level of clinical disease after virus challenge. However, the individuals in the negative control group experienced more severe clinical signs for a longer period of time than those in the rH_EIV inoculated group (Figure 5B). The H3 specific antibody response upon challenge was also determined. As seen in Figure 6, dogs in the group vaccinated with the rH_EIV vector vaccine showed an approximately 10-fold higher increase in HI titers following challenge infection at 7dpi compared to the mock-vaccinated group. The differences in titers were also apparent on day 14 pi, but differences were reduced to 4-fold over the mock-vaccinated group.
Figure 5. Immunization of dogs with rH_EIV provides partial clinical protection against challenge with virulent CIV.
Two groups of four dogs were vaccinated s.c. twice with rH_EIV or mock-vaccinated. All dogs were challenged 3 weeks after booster vaccination with 1×106 PFU A/canine/PA/10915-07 and physical exams were performed on days 0–8, 10, and 15. The clinical score given to each individual was based on several clinical signs, as described under Mat. & Meth (A). Body temperatures were also measured in these dogs and fever was defined as a rectal temperature of ≥ 39.4°C (B). Asterisks indicate statistically significant differences.
Figure 6. Immunization of dogs with rH_EIV results in a 10-fold increase in neutralizing antibodies upon challenge with virulent CIV.
Two groups of four dogs were vaccinated s.c. twice with rH_EIV or mock-vaccinated. All dogs were challenged 3 weeks after booster vaccination with 1x106 PFU A/canine/PA/10915-07 and serum was collected on days 0, 7 and 14. Serum neutralizing antibodies were determined by a standard hemagglutinin inhibition (HI) assay.
Finally, we investigated the amount and duration of CIV shedding in nasal swabs of dogs from both groups upon challenge. The amount of detectable virus found in nasal swabs using qRT-PCR was higher (by approximately a factor of 10) in the negative control group than in the vaccinated group (statistically significant differences were seen between 4 and 6 dpi) and the duration of virus shedding was reduced by approximately two days in the vaccinated group (Figure 7A). Analysis of virus titers by TCID50 was determined by inoculation of nasal swabs into MDCK cells (Figure 7B) and eggs (Figure 7C), and supported the qRT-PCR findings where RNA copies were determined. TCID50 analysis of virus titers showed that the unvaccinated control group was found to shed a significantly greater amount of virus for a longer period of time (Figure 7B, p<0.05). While virus could be isolated from all animals of the mock-vaccinated group for as long as 6 dpi, virus could be recovered only at day 1 and 2 post challenge in all four animals of the vaccinated group (Figure 7C). Moreover, virus could still be isolated in 50% of the mock-vaccinated animals at 8 dpi, whereas only 25% of the animals in the vaccinated group showed virus shedding at 5 dpi and by day 7 post challenge, no virus could be recovered from any of these animals (Figure 7C). From the results of the vaccination-challenge experiment, we concluded that at least partial protection against infection with a recent and highly virulent CIV strain could be induced in the natural host, using EHV-1 as a vector delivering the H3 immunogen.
Figure 7. Immunization of dogs with rH_EIV results in a reduction in amount and duration of viral shedding upon challenge with virulent CIV.
Two groups of four dogs were vaccinated s.c. twice with rH_EIV or mock-vaccinated. All dogs were challenged 3 weeks after booster vaccination with 1×106 PFU A/canine/PA/10915-07 and nasal swabs were taken on days 0–8, 10, and 15. RT-PCR was used to determine virus titers based on the number of influenza RNA copies (A). Virus isolation was performed by inoculating swab fluids into MDCK cells (B) and the presence or absence of virus was also determined by inoculation of eggs (C). Asterisks indicate statistically significant differences.
Discussion
Canine influenza virus (CIV) has only been recently described as a pathogen in dogs and emerged from an interspecies transfer of the H3N8 equine influenza virus. This was first recognized as the cause of significant disease in greyhounds [2]. Over the past few years CIV has spread widely throughout the USA and has infected dogs from various breeds, indicating that CIV has likely become enzootic among the dog population, at least in the USA. Moreover, as dogs are amongst the most common companion animals for humans, it is conceivable that they might become a source of influenza transmission to humans, now that the general transmissibility of influenza viruses to dogs under natural conditions has been demonstrated.
In general, both vaccination and management regimens are critical for prevention and control of influenza virus infections. To improve influenza vaccines, new strategies focus on the development of vaccines that closely mimic natural infection, as those are expected to provide the best protection. Currently, the use of modified live vector vaccines (MLV) expressing synthetic, codon-optimized antigens which are copies of the naturally encoded genes, are being intensively studied. These have been evaluated in several species for their potential to stimulate both humoral and cell-mediated immune responses against influenza virus [17, 20–22]. Viral vectors may be preferred vehicles for heterologous gene delivery as they can induce similar immune responses to those observed during natural infection. Upon entry of the viral vector into cells, antigens will be processed and presented through classical antigen presenting pathways, e.g. MHC class I, thereby not only resulting in humoral but also cytotoxic T cell responses, which will lead to stronger, more effective and longer-lasting immunity [23].
In this study, we have constructed and characterized a recombinant EHV-1 vector expressing the haemagglutinin (HA) H3 of A/equine/Ohio/03. The HA is essential for viral attachment and entry into cells and is also a major target for protective immune responses, and therefore, an ideal candidate for use in flu vaccination strategies [24]. The recombinant vector, rH_EIV, retained robust and stable expression of H3 in all infected cells for ten passages in tissue culture. Since no reduction in expression levels were observed, we surmise that protein expression stays robust for extended passaging of the vaccine, an important quality feature of a vectored vaccine. The growth properties of rH_EIV were comparable to those of the parental virus, indicating that insertion of H3 in RacH does not significantly interfere with the replication of EHV-1 in vitro. Interestingly, rH_EIV grew slightly better than parental HΔgp2 virus, as demonstrated by an approximately 2- to 3-fold increase in virus titers at various time points. The robust protein expression of rH_EIV and the vigorous growth properties will likely make this vectored vaccine an easily manageable candidate for vaccine production.
Humoral antibody responses against HA are considered good indicators for evaluating potentially protective immunity upon natural or vaccine-induced influenza infection [24, 25]. An increase in the antibody response against this influenza antigen is highest upon natural infection, moderate after vaccination with MLV and least effective after immunization with inactivated preparations [26]. However, the induction of cytotoxic T cell responses and memory CD8+ cells are considered important for protection as well, and, in fact, the first successful DNA vaccination against a virus was shown with the influenza virus nucleoprotein in mice [27]. Here we show that following inoculation with our MLV rH_EIV, H3-specific immune responses were induced in both mice and dogs. In mice, different concentrations of rH_EIV were tested and the HI titers were clearly dose-dependent with the highest upon intranasal immunization with 1×105 PFU rH_EIV. This is consistent with what has been observed previously, namely that antibody titers are directly related to the dose of antigen and frequency of antigenic stimulation [26]. In dogs, a subcutaneous inoculation, performed twice in a 4-week interval, induced a robust antibody response against CIV and antibodies could be readily detected within two weeks after the first immunization using a sensitive competitive ELISA. The HI antibody response, which is viewed as a good indicator of protection [24, 25], was detected in all immunized dogs after booster vaccination.
In addition to the immunization studies, an intranasal challenge infection experiment was carried out in dogs using a highly virulent CIV strain, A/canine/PA/10915-07. The antibody titers induced by the vectored vaccine increased rapidly upon challenge and this booster effect was approximately 4-fold higher in immunized dogs compared to the non-immunized control animals, indicating a strong memory response. An HI study of serum samples obtained from greyhounds with respiratory disease and asymptomatic contacts during and after the Florida outbreak in 2004 showed that CIV spread rapid and efficient among dogs, mainly via respiratory secretions [2]. Therefore, a successful vaccine must achieve a reduction in viral shedding by diminishing clinical signs such as nasal discharge and coughing. Dogs immunized with rH_EIV exhibited less severe clinical signs and also shed significantly less virus following challenge. The vaccination protocol presented here with the rH_EIV vector vaccine clearly showed partial clinical and virological protection against challenge infection with a virulent strain of CIV. Future studies are planned to increase the immunogenicity of the vectored vaccine in order to obtain complete protection against CIV by examining the effects of vaccine virus doses, the number of vaccinations and the time intervals between applications, as well as, and most importantly, different routes of administration. Preferably, local immunity should be induced at the site of natural infection, and it has been reported that administration of vaccines against respiratory infections via the nasal route is more efficacious than when applied parentally as the local nasal and upper respiratory tract immune system is stimulated [28]. For human influenza virus, the efficacy of several, so-called nasal vaccines have been extensively evaluated. A cold-adapted, live attenuated nasal influenza virus vaccine was confirmed to be safe and efficacious in humans because of the induction of secretory nasal IgA (sIgA) as well as IFN-γ production by specific CD4+ T-cells, even though only a low specific IgG response in serum was observed [29]. Intranasal vaccination with an inactivated trivalent influenza vaccine was also shown to be effective in humans and was able to induce both mucosal sIgA, as well as serum IgG immunologic responses [30]. It might be of interest to evaluate the efficacy of the rH_EIV vaccine upon intranasal administration of our vectored canine influenza vaccine, since EHV-1 naturally targets the nasal mucosa. In addition, the vaccine might also benefit from insertion of other important influenza proteins like e.g. the nucleoprotein [27]. Although such a vectored combination vaccine would first need to be fully re-characterized for proper growth in vitro and expression, we do not foresee major problems since EHV-1 is known to accommodate large amounts of foreign DNA [14].
The effects of rH_EIV inoculation on local mucosal or systemic cell-mediated immune responses were not investigated, but rH_EIV appears to be a good stimulator of a protective immunity against canine influenza and can aid in reducing transmission of CIV to allow a better control of the disease. As a result of the conservative design of the dog experiment, which did not include a vector-only control, we cannot formally exclude that innate and unspecific responses not directed to H3 contribute to the protection conferred by the EHV-1 recombinant virus. Based on the notion that herpesviruses in general and EHV-1 in particular will dampen and subvert rather than induce innate responses, and based on experiments using RacH-derived modified live virus vaccines in horses, bovines and mice, we view the induction of an unspecific protective response as highly unlikely [11–14]. In addition and as discussed earlier, the rH_EIV vector induced a robust H3-specific antibody response as measured by ELISA and HI. Such a response is regarded as a good correlate of protection, which, in our opinion, supports the notion that specific rather than unspecific responses are responsible for the reduction of clinical signs in vaccinated and challenge-infected dogs.
Taken together, we here report the first vaccination-challenge study evaluating the protective efficacy of any vaccine formulation against CIV. Our results clearly show that recombinant EHV-1 expressing H3 can efficiently deliver the major influenza virus immunogen to dogs and induce a protective immune response against CIV. This protective immune response partially protects dogs from clinical signs like fever and coughing, and is able to reduce viral shedding upon infection with severe CIV. We are planning future studies, which shall concentrate on comparative efficacy studies using different (recombinant) vaccines and routes of application.
Acknowledgments
The authors are grateful to Dr. Judy Appleton for providing anti-H3 antibodies and Dr. Robin Gleed for help in developing the custom-engineered nebulizer. We thank Dr. Douglas F. Antczak for his continued support of the study. This work was supported in part by the PHS grant 5R21AI061412, R01GM080533, and by the Harry M. Zweig fund for equine research awarded to N.O., and funds made available through the Baker Institute for Animal Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Neumann G, Kawaoka Y. Host range restriction and pathogenicity in the context of influenza pandemic. Emerg.Infect.Dis. 2006;12(6):881–886. doi: 10.3201/eid1206.051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford PC, Dubovi EJ, Castleman WL, et al. Transmission of equine influenza virus to dogs. Science. 2005;310(5747):482–485. doi: 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- 3.Paillot R, Hannant D, Kydd JH, Daly JM. Vaccination against equine influenza: quid novi? Vaccine. 2006;24(19):4047–4061. doi: 10.1016/j.vaccine.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 4.van Maanen C, van Essen GJ, Minke J, Daly JM, Yates PJ. Diagnostic methods applied to analysis of an outbreak of equine influenza in a riding school in which vaccine failure occurred. Vet.Microbiol. 2003;93(4):291–306. doi: 10.1016/s0378-1135(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 5.Animal Health Diagnostic Center. Emerging Issues, D.L. W. s. 2007 (GENERIC) Ref Type: Internet Communication. [Google Scholar]
- 6.Patel JR, Heldens J. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)--epidemiology, disease and immunoprophylaxis: a brief review. Vet.J. 2005;170(1):14–23. doi: 10.1016/j.tvjl.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 7.van Maanen C. Equine herpesvirus 1 and 4 infections: an update. Vet.Q. 2002;24(2):58–78. [PubMed] [Google Scholar]
- 8.Hubert PH, Birkenmaier S, Rziha HJ, Osterrieder N. Alterations in the equine herpesvirus type-1 (EHV-1) strain RacH during attenuation. Zentralbl.Veterinarmed.B. 1996;43(1):1–14. doi: 10.1111/j.1439-0450.1996.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer A, Meindl A, Osterrieder N. Mutations in the US2 and glycoprotein B genes of the equine herpesvirus 1 vaccine strain RacH have no effects on its attenuation. Berl Munch.Tierarztl.Wochenschr. 1999;112(9):351–354. [PubMed] [Google Scholar]
- 10.Osterrieder N, Hubert PH, Brandmuller C, Kaaden OR. A touchdown PCR for the differentiation of equine herpesvirus type 1 (EHV-1) field strains from the modified live vaccine strain RacH. J.Virol.Methods. 1994;50(1–3):129–136. doi: 10.1016/0166-0934(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 11.Rosas CT, Goodman LB, von Einem J, Osterrieder N. Equine herpesvirus type 1 modified live virus vaccines: quo vaditis? Expert.Rev.Vaccines. 2006;5(1):119–131. doi: 10.1586/14760584.5.1.119. [DOI] [PubMed] [Google Scholar]
- 12.Rosas CT, Konig P, Beer M, Dubovi EJ, Tischer BK, Osterrieder N. Evaluation of the vaccine potential of an equine herpesvirus type 1 vector expressing bovine viral diarrhea virus structural proteins. J.Gen.Virol. 2007;88(Pt 3):748–757. doi: 10.1099/vir.0.82528-0. [DOI] [PubMed] [Google Scholar]
- 13.Rosas CT, Tischer BK, Perkins GA, Wagner B, Goodman LB, Osterrieder N. Live-attenuated recombinant equine herpesvirus type 1 (EHV-1) induces a neutralizing antibody response against West Nile virus (WNV) Virus Res. 2007;125(1):69–78. doi: 10.1016/j.virusres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Trapp S, von Einem J, Hofmann H, et al. Potential of equine herpesvirus 1 as a vector for immunization. J.Virol. 2005;79(9):5445–5454. doi: 10.1128/JVI.79.9.5445-5454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph J, Osterrieder N. Equine herpesvirus type 1 devoid of gM and gp2 is severely impaired in virus egress but not direct cell-to-cell spread. Virology. 2002;293(2):356–367. doi: 10.1006/viro.2001.1277. [DOI] [PubMed] [Google Scholar]
- 16.Tischer BK, von Einem J, Kaufer B, Osterrieder N. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques. 2006;40(2):191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- 17.Karaca K, Dubovi EJ, Siger L, et al. Evaluation of the ability of canarypox-vectored equine influenza virus vaccines to induce humoral immune responses against canine influenza viruses in dogs. Am.J.Vet.Res. 2007;68(2):208–212. doi: 10.2460/ajvr.68.2.208. [DOI] [PubMed] [Google Scholar]
- 18.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J.Clin.Microbiol. 1999;37(4):937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spackman E, Senne DA, Myers TJ, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J.Clin.Microbiol. 2002;40(9):3556–3560. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bublot M, Pritchard N, Swayne DE, et al. Development and use of fowlpox vectored vaccines for avian influenza. Ann.N.Y.Acad.Sci. 2006;1081:193–201. doi: 10.1196/annals.1373.023. [DOI] [PubMed] [Google Scholar]
- 21.Minke JM, Toulemonde CE, Dinic S, Cozette V, Cullinane A, Audonnet JC. Effective priming of foals born to immune dams against influenza by a canarypox-vectored recombinant influenza H3N8 vaccine. J.Comp Pathol. 2007;137 Suppl 1:S76–S80. doi: 10.1016/j.jcpa.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Minke JM, Toulemonde CE, Coupier H, et al. Efficacy of a canarypox-vectored recombinant vaccine expressing the hemagglutinin gene of equine influenza H3N8 virus in the protection of ponies from viral challenge. Am.J.Vet.Res. 2007;68(2):213–219. doi: 10.2460/ajvr.68.2.213. [DOI] [PubMed] [Google Scholar]
- 23.Brave A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol.Pharm. 2007;4(1):18–32. doi: 10.1021/mp060098+. [DOI] [PubMed] [Google Scholar]
- 24.Schild GC, Oxford JS, Virelizier JL. Immunity to influenza. Dev.Biol.Stand. 1975;28:253–272. [PubMed] [Google Scholar]
- 25.Couch RB, Kasel JA. Immunity to influenza in man. Annu.Rev.Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 26.Naikhin AN, Artem'eva SA, Ermachenko TA, et al. Antibody functional activity in immunization with influenza vaccines. Vopr.Virusol. 1993;38(5):204–207. [PubMed] [Google Scholar]
- 27.Montgomery DL, Shiver JW, Leander KR, et al. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12(9):777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 28.Zuercher AW. Upper respiratory tract immunity. Viral Immunol. 2003;16(3):279–289. doi: 10.1089/088282403322396091. [DOI] [PubMed] [Google Scholar]
- 29.Tomoda T, Morita H, Kurashige T, Maassab HF. Prevention of influenza by the intranasal administration of cold-recombinant, live-attenuated influenza virus vaccine: importance of interferon-gamma production and local IgA response. Vaccine. 1995;13(2):185–190. doi: 10.1016/0264-410x(95)93134-u. [DOI] [PubMed] [Google Scholar]
- 30.Greenbaum E, Furst A, Kiderman A, et al. Mucosal [SIgA] and serum [IgG] immunologic responses in the community after a single intra-nasal immunization with a new inactivated trivalent influenza vaccine. Vaccine. 2002;207(7–8):1232–1239. doi: 10.1016/s0264-410x(01)00396-6. [DOI] [PubMed] [Google Scholar]