Abstract
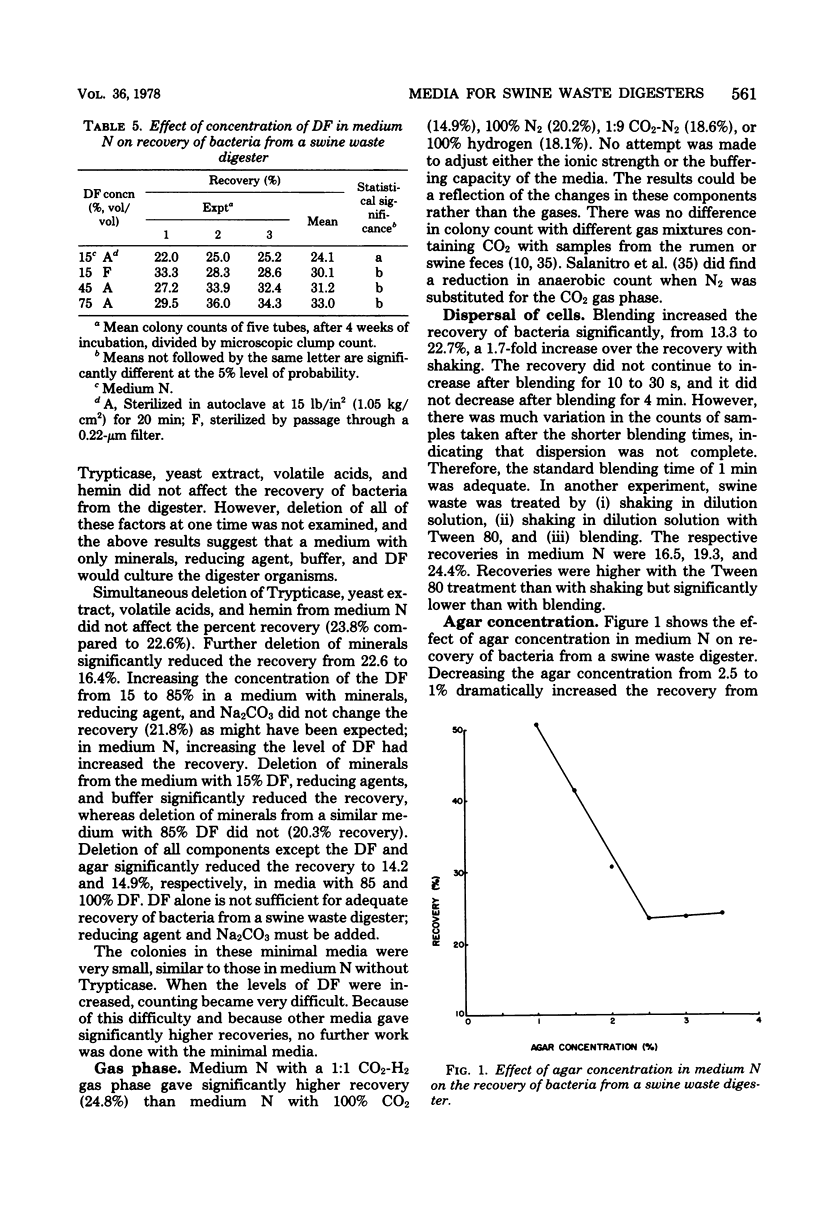
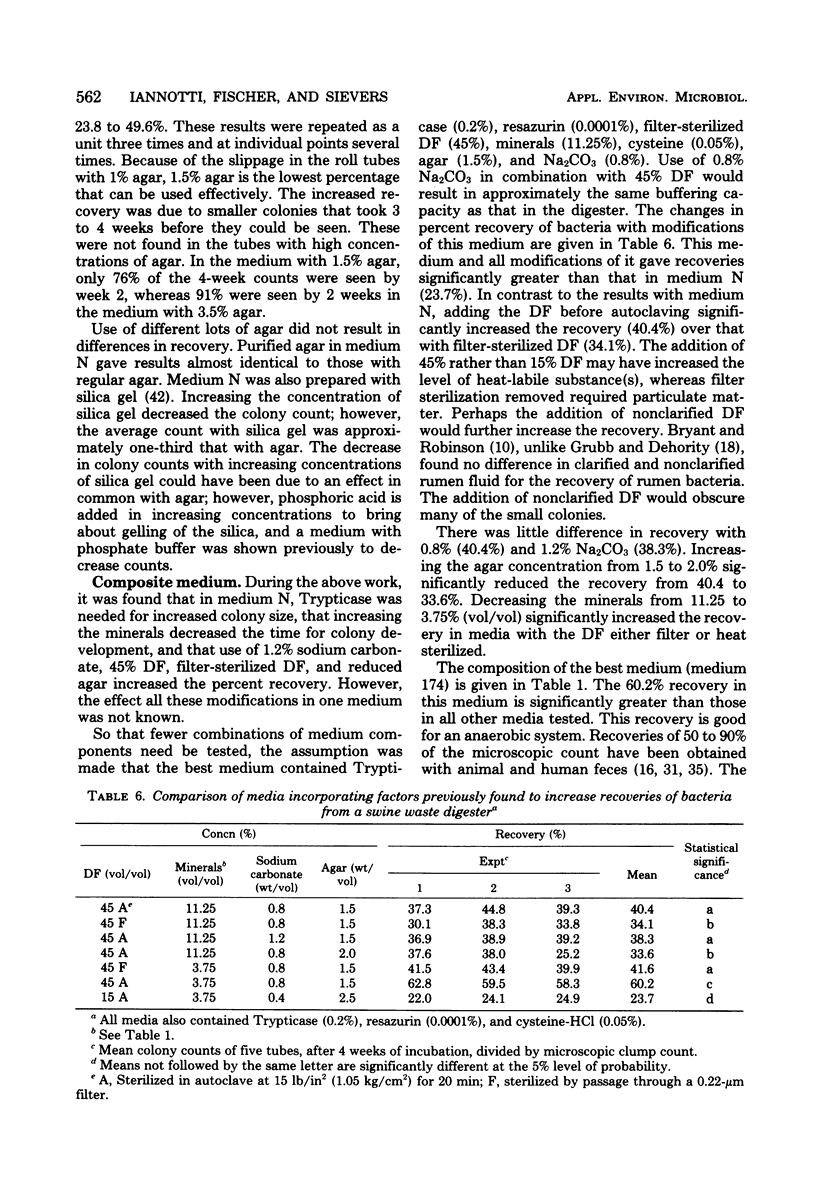
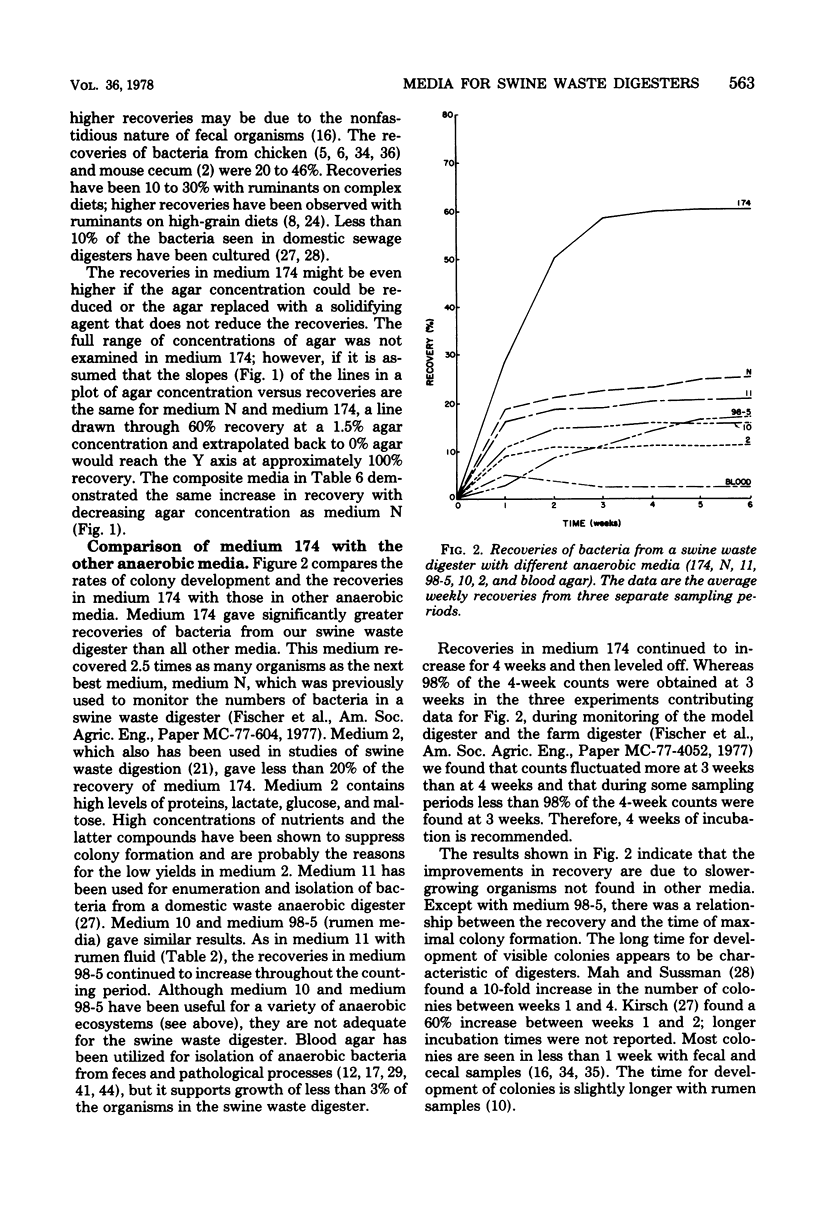
A habitat-simulating medium was developed for the enumeration and isolation of bacteria from a swine waste digester. A roll tube medium with growth factors for strict anaerobes from previously studied anaerobic ecosystems was used to evaluate the effects of deletion, addition, or level of digester fluid, digester fluid treated with acid or base, rumen fluid, fecal extract, anaerobic pit extract, tissue extract, carbohydrates, peptones, short-chain fatty acids, minerals, vitamins, N and P sources, reducing and solidifying agents, buffers, and gases on colony counts. Decreasing the agar concentration from 2.5 to 1.0% increased the counts twofold. Blending increased the counts 1.7-fold. With a medium (174) containing digester fluid, peptones, minerals, cysteine, sodium carbonate, and agar, colony counts were 60% of the microscopic count and improved yields 2.5 to 20 times those obtained with media previously used for digesters or developed for other anaerobic ecosystems. Colony counts continued to increase for up to 4 weeks of incubation. Medium 174 permits the enumeration of total, methanogenic, and, with deletion of reducing agent, aerotolerant bacteria. The results suggest that the predominant bacteria grow slowly and have requirements different from those of bacteria from other ecosystems.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Burton G. C. The effect of hibernation on the caecal flora of the thirteen-lined ground squirrel (Citellus tridecemlineatus). J Appl Bacteriol. 1970 Sep;33(3):505–514. doi: 10.1111/j.1365-2672.1970.tb02227.x. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Impey C. S. The isolation and properties of the predominant anaerobic bacteria in the caeca of chickens and turkeys. Br Poult Sci. 1970 Oct;11(4):467–481. doi: 10.1080/00071667008415842. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Mead G. C., Barnum D. A., Harry E. G. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci. 1972 May;13(3):311–326. doi: 10.1080/00071667208415953. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., McBride B. C., Wolfe R. S. Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J Bacteriol. 1968 Mar;95(3):1118–1123. doi: 10.1128/jb.95.3.1118-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. T., Fulk G. E., Silverman S. J. Dietary effects on the composition of fecal flora of rats. Appl Environ Microbiol. 1977 Mar;33(3):654–659. doi: 10.1128/aem.33.3.654-659.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A., Grubb J. A. Basal medium for the selective enumeration of rumen bacteria utilizing specific energy sources. Appl Environ Microbiol. 1976 Nov;32(5):703–710. doi: 10.1128/aem.32.5.703-710.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T., McBride B. C. New method for the isolation and identification of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):540–545. doi: 10.1128/am.29.4.540-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller C., Crabill M. R., Bryant M. P. Anaerobic roll tube media for nonselective enumeration and isolation of bacteria in human feces. Appl Microbiol. 1971 Oct;22(4):522–529. doi: 10.1128/am.22.4.522-529.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M., Attebery H. R., Sutter V. L. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am J Clin Nutr. 1974 Dec;27(12):1456–1469. doi: 10.1093/ajcn/27.12.1456. [DOI] [PubMed] [Google Scholar]
- Grubb J. A., Dehority B. A. Variation in colony counts of total viable anaerobic rumen bacteria as influenced by media and cultural methods. Appl Environ Microbiol. 1976 Feb;31(2):262–267. doi: 10.1128/aem.31.2.262-267.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. A., Reddy C. A., Carter G. R. Anaerobic bacteria from the large intestine of mice. Appl Environ Microbiol. 1976 Jun;31(6):907–912. doi: 10.1128/aem.31.6.907-912.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Sussman C. Microbiology of anaerobic sludge fermentation. I. Enumeration of the nonmethanogenic anaerobic bacteria. Appl Microbiol. 1968 Feb;16(2):358–361. doi: 10.1128/am.16.2.358-361.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B. R., Flynn M. A., Burton G. C., Tsutakawa R. K., Hentges D. J. Effects of a high-beef diet on bowel flora: a preliminary report. Am J Clin Nutr. 1974 Dec;27(12):1470–1474. doi: 10.1093/ajcn/27.12.1470. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham P. M., Hungate R. E. Isolation of methanogenic bacteria from feces of man. J Bacteriol. 1968 Dec;96(6):2178–2179. doi: 10.1128/jb.96.6.2178-2179.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynter M. J., Hungate R. E. Characterization of Methanobacterium mobilis, sp. n., isolated from the bovine rumen. J Bacteriol. 1968 May;95(5):1943–1951. doi: 10.1128/jb.95.5.1943-1951.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. H., HUNGATE R. E. Isolation and characterization of Methanobacterium ruminantium n. sp. J Bacteriol. 1958 Jun;75(6):713–718. doi: 10.1128/jb.75.6.713-718.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirhead P. A. Isolation and identification of fecal bacteria from adult swine. Appl Environ Microbiol. 1977 Jan;33(1):79–84. doi: 10.1128/aem.33.1.79-84.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirhead P. A. Studies on the cecal microflora of commercial broiler chickens. Appl Microbiol. 1974 Sep;28(3):439–447. doi: 10.1128/am.28.3.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Fairchilds I. G., Zgornicki Y. D. Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Appl Microbiol. 1974 Apr;27(4):678–687. doi: 10.1128/am.27.4.678-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears R. W., Freter R. Improved isolation of anaerobic bacteria from the mouse cecum by maintaining continuous strict anaerobiosis. Proc Soc Exp Biol Med. 1967 Mar;124(3):903–909. doi: 10.3181/00379727-124-31882. [DOI] [PubMed] [Google Scholar]
- Thatcher R. C., Weaver T. L. Simplified method for the preparation of silica gel media. Appl Microbiol. 1974 Nov;28(5):887–888. doi: 10.1128/am.28.5.887-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J. Studies of the cultivable flora of normal human feces. Antonie Van Leeuwenhoek. 1966;32(2):212–222. doi: 10.1007/BF02097463. [DOI] [PubMed] [Google Scholar]


