Abstract
Rotary catalysis in F1F0 ATP synthase is powered by proton translocation through the membrane-embedded F0 sector. Proton binding and release occurs in the middle of the membrane at Asp-61 on transmembrane helix 2 of subunit c. Previously, the reactivity of cysteines substituted into F0 subunit a revealed two regions of aqueous access, one extending from the periplasm to the middle of the membrane and a second extending from the middle of the membrane to the cytoplasm. To further characterize aqueous accessibility at the subunit a-c interface, we have substituted Cys for residues on the cytoplasmic side of transmembrane helix 2 of subunit c and probed the accessibility to these substituted positions using thiolate-reactive reagents. The Cys substitutions tested were uniformly inhibited by Ag+ treatment, which suggested widespread aqueous access to this generally hydrophobic region. Sensitivity to N-ethylmaleimide (NEM) and methanethiosulfonate reagents was localized to a membrane-embedded pocket surrounding Asp-61. The cG58C substitution was profoundly inhibited by all the reagents tested, including membrane impermeant methanethiosulfonate reagents. Further studies of the highly reactive cG58C substitution revealed that NEM modification of a single c subunit in the oligomeric c-ring was sufficient to cause complete inhibition. In addition, NEM modification of subunit c was dependent upon the presence of subunit a. The results described here provide further evidence for an aqueous-accessible region at the interface of subunits a and c extending from the middle of the membrane to the cytoplasm.
F1F0 ATP synthase utilizes the energy stored in an H+ or Na+ electrochemical gradient to synthesize ATP in bacteria, mitochondria, and chloroplasts (1–3). The ATP synthase complex is composed of two sectors, i.e. a water-soluble F1 sector that is bound to a membrane-embedded F0 sector. In bacteria F1 is composed of five subunits in a α3β3γδε ratio and contains three catalytic sites for ATP synthesis and/or hydrolysis centered at the α/β subunit interfaces (4). F0 is composed of three subunits in a a1b2c10–15 ratio and acts as the ion channel (3–8). Ion translocation through F0 drives rotation of a cylindrical ring of c-subunits which is coupled to rotation of the γ subunit within the (αβ)3 hexamer of F1 to force conformational changes in the three active sites and catalyze synthesis of ATP by the binding change mechanism (9–11).
Subunit c of F0 folds in the membrane as a hairpin of two extended α helices. In Escherichia coli, 10 copies of subunit c pack together to form a decameric ring with TMH12 on the inside and TMH2 on the periphery (5, 12). A recent high resolution structure of the Na+-translocating c11-ring from Ilyobacter tartaricus defined the Na+ binding site formed between two interacting c subunits (13). The essential Na+ binding Glu residue, which corresponds to Asp-61 in E. coli, lies on TMH2 in the middle of the lipid bilayer. Subunit a consists of five transmembrane helices, four of which likely interact as a four helix bundle (14, 15). Subunit a lies on the periphery of the c-ring with TMHs 4 and 5 from subunit a and TMH2 from subunit c forming the a-c interface (15, 16). During ion translocation through F0, the essential Arg-210 on TMH4 of subunit a is postulated to facilitate the protonation and deprotonation of Asp-61 of subunit c and cause the rotation of the c-ring past the stationary subunit a (16).
Cysteine substitution mutagenesis has been widely used to probe aqueous accessible positions in transmembrane proteins (17). The reactivity of a substituted cysteine to thiolate-directed probes provides an indication of aqueous accessibility since the highly reactive thiolate form is preferentially formed in an aqueous environment. The method has been applied previously to the alternating access model of LacY using N-ethylmaleimide as a probe (18) and to map pore-forming residues in a K+ channel using silver ion as a probe (19). The aqueous accessibility of the five TMHs in subunit a of E. coli F0 have been probed using silver ion and NEM. The results suggest the presence of an aqueous accessible channel in subunit a in the center of TMHs 2–5 extending from the periplasm to the center of the membrane (20–22). Protons entering through this periplasmic access channel are postulated to bind to the essential Asp-61 residues of the c-ring, and the protonated subunit is then carried through a full rotation before the protons exit by a still uncertain pathway to the cytoplasm.
During H+-driven ATP synthesis, two models for the cytoplasmic exit pathway for H+ have been proposed. The models alternatively propose that the ions exit to the cytoplasm either through a second half-channel in subunit a (20–22) or by an aqueous channel that is intrinsic to the c subunit (3). Studies of the c-ring from the I. tartaricus enzyme indicate that Na+ can access Glu-65 in the absence of other F0 subunits, suggesting an intrinsic channel in subunit c (23–24). However, no such channel was apparent in the crystal structure of the c11 ring (13). Chemical modification studies of Cys-substituted subunit a of E. coli revealed an aqueous-accessible surface of TMH4, including the essential Arg-210 residue, which extended from the center of the membrane to the cytoplasm and suggested that the ion exit channel may lie at the a-c interface (20). To further define the role of subunit c in providing aqueous access to the cytoplasm, we have extended our probing of thiolate reactivity of substituted Cys to TMH2 of subunit c using various thiolate-reactive reagents. Despite the hydrophobicity of the region, a large proportion of the substituted Cys from M65C at the center of the membrane to the cytoplasmic membrane surface proved to be reactive with Ag+. Furthermore, reagent access to at least one position in this membrane-embedded region of subunit c was facilitated by subunit a. The experiments provide more information on the aqueous pathway by which H3O+ may be transported through F0 and provide additional evidence that subunit a participates directly in the ion exit pathway from cAsp-61 at the center of the membrane to the cytoplasm.
EXPERIMENTAL PROCEDURES
Mutagenesis—A two-step PCR method that utilizes a mutagenic primer and two wild type primers (25) was used to generate the cysteine substitutions in subunit c. The template plasmid, pCMA113, codes the eight structural genes of F1F0, wherein all endogenous cysteines are replaced with alanine or serine and subunit a is modified with a C-terminal His tag (20). Mutant PCR fragments were transferred into pCMA113 between the BsrGI and BssHII (or PpuMI) restriction sites. The presence of each substitution was confirmed by DNA sequencing through the ligation sites. An in-frame deletion of subunit a (uncB) was combined with the cG58C substitution by amplifying the deletion region from the chromosomal uncB deletion strain VF245 (26). The amplified fragment containing the deletion was then inserted into the pCMA113 plasmid between the HindIII and BsrGI restriction sites. The presence of the ΔuncB deletion and the cG58C substitution were confirmed by DNA sequencing through the deletion fragment ligation sites and the uncE gene encoding G58C subunit c. All mutant plasmids were transferred into the chromosomal unc operon deletion strain JWP292 (5). Growth yields of the mutant strains on glucose minimal medium and succinate minimal medium agar plates were assayed as described previously (20).
Membrane Preparation—JWP292 transformant strains were grown in M63 minimal medium containing 0.6% glucose, 0.2 mm uracil, 1 mm arginine, 2 μg/ml thiamine, 40 μm 2,3-dihydroxybenzoic acid, 100 μg/ml ampicillin, and 10% LB broth. Cells were harvested by centrifugation in the late exponential stage of growth. Cell pellets were suspended in TMG acetate (50 mm Tris acetate, 5 mm magnesium acetate, 10% glycerol, pH 7.5) supplemented with 1 mm dithiothreitol, 1 mm phenylmethanesulfonyl fluoride, and 0.1 mg/ml deoxyribonuclease I and disrupted by passage through a French press at 108 nt/m2. Cell debris was cleared by low speed centrifugation of the lysate at 7700 × gmax, and membranes were collected by high speed centrifugation of the cleared lysate at 193,000 × gmax. Membranes were washed once with TMG acetate and then resuspended in TMG acetate and stored at -80 °C. Protein concentrations were determined using a modified Lowry assay (27).
ATP-driven ACMA Fluorescence Quenching—Aliquots of membranes prepared from JWP292 transformant strains (1.6 mg at 10 mg/ml) were suspended in 3.2 ml HMK buffer (10 mm HEPES-KOH, 1 mm Mg(NO3)2, 10 mm KNO3, pH 7.5) and incubated at room temperature for 10 min. ACMA was added to 0.3 μg/ml, and fluorescence quenching was initiated by the addition of 30 μl of 25 mm ATP, pH 7. Each experiment was terminated by adding nigericin to 0.5 μg/ml. The restored level of fluorescence after the addition of nigericin was used as the base-line fluorescence from which the relative quenching of the mutant membranes was calculated. For treatment with silver, AgNO3 was added to the HMK buffer to 40 μm before the addition of membranes. For treatment with NEM, NEM was added to a 1.6-mg aliquot of membranes in 160 μl of TMG acetate buffer to a final concentration of 5 mm. After a 10-min incubation at room temperature, treated membranes were diluted into HMK buffer, and the quenching reaction was carried out as described above. Treatments with MTS derivatives were performed as for NEM treatment using variable concentrations and incubation times. Solutions of MTS derivatives were dissolved immediately before use in DMSO solvent. DMSO concentration ranged from 2.5 to 5% during the incubation period and from 0.1 to 0.2% during the measurement of fluorescence.
[14C]NEM Labeling of Subunit c—Membranes prepared from JWP292 transformant strains were diluted to 33 mg/ml in TMG acetate, pH 7.5, to a final volume of 3 ml. A 50-μCi aliquot of N-[14C]ethylmaleimide (PerkinElmer Life Sciences, specific activity = 33.8 mCi/mmol) was transferred from pentane solution into 200 μl of TMG acetate, pH 7.5, by mixing the pentane solution with the buffer, allowing the phases to separate and evaporating the pentane upper phase under a stream of argon. Membranes were labeled with 50 μCi of [14C]NEM at a final concentration of 0.51 mm. The labeling reaction was stopped after 15 min at room temperature by the addition of β-mercaptoethanol to 10 mm and unlabeled NEM to 20 mm.
Purification of Subunit c—Subunit c was purified from labeled membranes as previously described (29) with some modifications. Labeled membranes were slowly mixed with 100 ml of CHCl3:CH3OH (2:1) at 4 °C for 1 h and then filtered through Whatman #1 filter paper. To the filtrate, one-fifth volume of H2O was added and mixed gently at 4 °C. The phases were allowed to separate overnight at 4 °C. The upper phase was removed by suction, and the phase interface was washed with 10 ml of theoretical upper phase mixture (CHCl3:CH3OH: H2O in proportion 3:47:48). One volume of CHCl3 was added to the lower phase with stirring while adding the minimal amount of methanol necessary to keep the solution clear. This solution was evaporated to dryness in a rotoevaporator at 37 °C. The dried residue was dissolved in CHCl3:CH3OH (2:1) and mixed with 4.5 volumes of chilled ethyl ether. After incubation overnight at -20 °C, the precipitate was collected by centrifugation and redissolved in CHCl3:CH3OH (2:1). This “proteolipid” solution was applied to a carboxymethyl cellulose cation exchange column equilibrated with 6 bed volumes of CHCl3: CH3OH (2:1). The column was then washed with 2 bed volumes of CHCl3:CH3OH (2:1) and one bed volume of CHCl3:CH3OH (1:1). Subunit c was eluted as a single peak with CHCl3:CH3OH: H2O (5:5:1). The subunit c concentration in pooled elution fractions was determined using a Lowry assay and the extinction coefficient calculated for purified subunit c previously (30).
Electrophoresis—Subunit c in CHCl3:CH3OH:H2O (5:5:1) was transferred to aqueous solution by precipitation in 10 volumes chilled acetone. The acetone precipitate was incubated in SDS sample buffer (50 mm Tris-HCl, 4% (w/v) SDS, 12% (w/v) glycerol, pH 8.0) for at least 1 h at room temperature before electrophoresis. SDS-PAGE was carried out in a Tricine-buffered system according to the method of Schaegger and von Jagow (31). Gels were stained with Coomassie Brilliant Blue, sealed in cellophane, and exposed to a phosphor storage screen for 48 h. Amounts of protein and radioactivity were quantitated using Scion Image.
ESI-TOF Mass Spectrometry—Purified subunit c in CHCl3: CH3OH:H2O (5:5:1) was directly injected into an Agilent LC/MSD TOF mass spectrometer.
RESULTS
Characterization of Cysteine Substitutions—Residues on the cytoplasmic half of cTMH2 were mutated to cysteine by oligonucleotide-directed mutagenesis using a plasmid wherein all endogenous cysteines in F1 were substituted with alanine and wherein the single cysteine in subunit b of F0 was substituted with serine. Wild type subunits a and c lack endogenous cysteine. The mutant plasmid was transferred into the E. coli strain JWP292 in which the unc (atp) operon encoding F1F0 was deleted from the chromosome. Most of the cysteine substitution mutants grew nearly as well as wild type on both glucose and succinate minimal media (Table 1). Inverted membrane vesicles from most of the cysteine substitution strains were functional in ATP-driven proton pumping as assayed by ACMA fluorescence quenching (Table 1). The L59C substitution abolished growth on succinate, the indicator used here for a functional oxidative phosphorylation system, and eliminated ATP-driven proton pumping. The F54C and I55C substitutions led to a puzzling phenotype in which the mutants grew well on glucose and succinate minimal media but failed to carry out ATP-driven proton pumping.
TABLE 1.
Characterization of cysteine substitution mutants
| Substitution | Colony size on succinatea | Growth yield on glucoseb | % Quenching with ATPc |
|---|---|---|---|
| mm | |||
| Q52C | 2.1 | 87 | 61 |
| F53C | 2.1 | 91 | 64 |
| F54C | 2.1 | 100 | 6 |
| I55C | 2.1 | 95 | 3 |
| V56C | 2.2 | 108 | 68 |
| M57C | 2.0 | 89 | 71 |
| G58C | 2.1 | 87 | 57 |
| L59C | 0.1 | 55 | ≤2 |
| V60C | 2.2 | 103 | 65 |
| D61C | 0 | 65 | ≤2 |
| A62C | 1.1 | 98 | 78 |
| I63C | 2.1 | 100 | 74 |
| P64Cd | 2.1 | 95 | 69 |
| M65C | 2.1 | 94 | 66 |
Average colony size of wild type is 2.2 mm
Expressed as % of wild type growth yield in 0.04% glucose liquid medium. Average growth yield of the Δunc background is 60 ± 5%
Average quenching of wild type is 70 ± 5%
Constructed with an A20P suppressor mutation
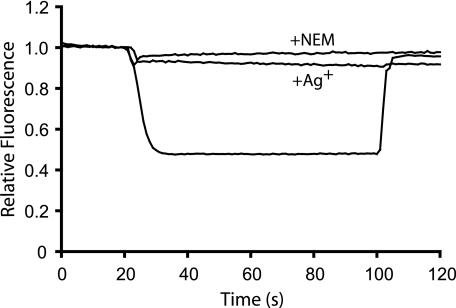
Reactivity of Cys Substitutions with Ag+ and NEM—We tested the aqueous accessibility of cysteines in positions 52–65 in subunit c using inverted membrane vesicles by examining the sensitivity of the ATP-driven quenching of ACMA fluorescence to inhibition by Ag+ and NEM. Mutants F54C, I55C, and L59C could not be tested due to the lack of function in this assay. Ag+ and NEM react preferentially with the thiolate form of the cysteine side chain, which should predominate in an aqueous versus a lipid exposed environment (19, 32, 33). Inhibition of proton pumping is thought to reflect obstruction of the proton translocation pathway on reaction of the reagent with the substituted cysteine (20). All 10 of the residues tested were strongly inhibited by 40 μm AgNO3 (Fig. 1 and Table 2). NEM inhibited proton pumping by the G58C mutant by >90% and proton pumping by the P64C and M65C mutants by ∼20% (Fig. 1 and Table 2). In experiments that are not shown with the G58C mutant, silver caused maximum inhibition within 30 s of treatment, and NEM inhibition reached a maximum within 5 min of treatment.
FIGURE 1.
Inhibition of ATP-driven proton pumping by Ag+ and NEM. ATP-driven quenching of ACMA fluorescence by cG58C-inverted membrane vesicles was assayed as described under “Experimental Procedures.” Fluorescence quenching was initiated by the addition of ATP at 20 s and terminated by the addition of nigericin at 100 s. The return to maximum fluorescence reached after the addition of nigericin was used to calculate the relative quenching values given in Table 1 and the inhibition values given in Table 2. Treatment with an inhibitor as described under “Experimental Procedures” is indicated.
TABLE 2.
Sensitivity of mutants to sulfhydryl-reactive reagents MTSM, MTS-methyl; MTSCE, MTS-carboxyethyl.
|
Substitution
|
Inhibition of ATP driven
quenchinga
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag+ | NEM | MTSM | MTSCE | MTSEA | MTSES | MTSET | |||||||
| % | |||||||||||||
| Q52C | 95 | ≤5 | ≤5 | 11 | ≤5 | 14 | 17 | ||||||
| F53C | 95 | ≤5 | ≤5 | 6 | 15 | 9 | 12 | ||||||
| V56C | 96 | ≤5 | 6 | 10 | ≤5 | 10 | 8 | ||||||
| M57C | 96 | 7 | ≤5 | ≤5 | 93 | 29 | 63 | ||||||
| G58C | 97 | 93 | 83 | 71 | 95 | 75 | 94 | ||||||
| V60C | 97 | ≤5 | ≤5 | ≤5 | 14 | ≤5 | 8 | ||||||
| A62C | 97 | ≤5 | 6 | ≤5 | 94 | ≤5 | ≤5 | ||||||
| I63C | 96 | ≤5 | ≤5 | ≤5 | 60 | ≤5 | ≤5 | ||||||
| P64Cb | 95 | 20 | ≤5 | 10 | 58 | 10 | 12 | ||||||
| M65C | 94 | 19 | ≤5 | 13 | 81 | 10 | 13 | ||||||
Each determination is the average of ≥2 trials. The average S.D. was 1% for Ag+ inhibition, 3% for NEM inhibition, and 10% for MTS inhibition. MTS reagents were tested at a final concentration of 1 mm
Constructed with an A20P suppressor mutation
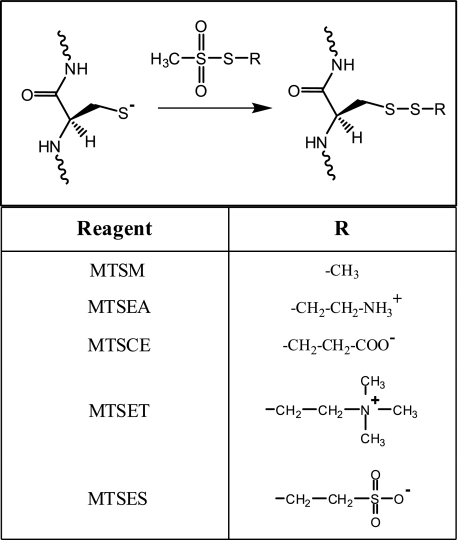
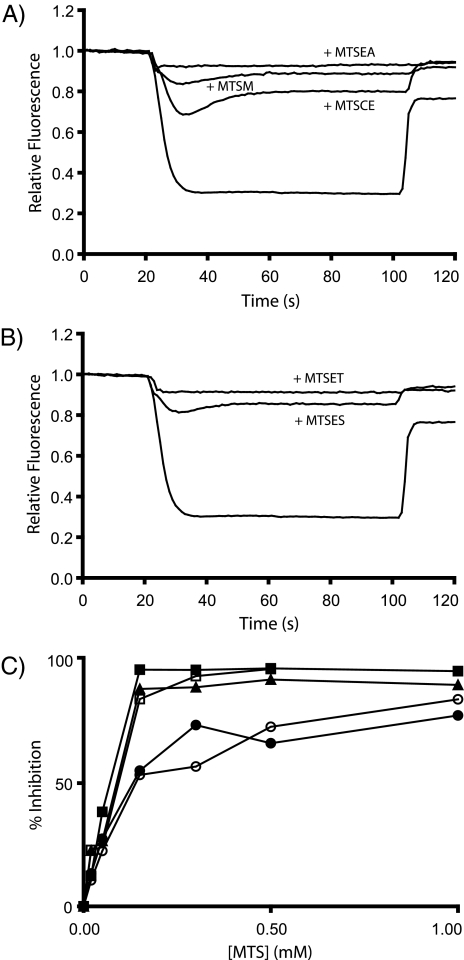
Reactivity of Cys Substitutions with MTS Reagents—MTS reagents react exclusively with the thiolate form of cysteine (34) and can be widely derivatized to make them versatile probes for aqueous accessibility. We probed the substituted Cys with the MTS derivatives shown in Fig. 2 by testing for inhibition of proton pumping as above. The results are summarized in Table 2. Maximum inhibition by MTS reagents occurred within 1 min of reagent addition, which is consistent with the published reactivity of these reagents with cysteines in other proteins (35, 36). Every derivative of MTS tested profoundly inhibited proton pumping by the G58C mutant (Fig. 3, A and B). MTSES and MTSET significantly inhibited proton pumping by both the M57C and G58C mutants and slightly inhibited proton pumping by the Q52C mutant. Inhibition by MTSES and MTSET in this region of the protein is of particular interest given that these reagents cannot cross liposomes or the E. coli inner membrane (35, 37). Therefore, MTSES and MTSET must be accessing residues 57 and 58 from the “cytoplasmic” side of the inverted vesicle. On testing inhibition of the cG58C mutant by MTS reagents over a range of concentrations, the positively charged reagents were found to cause greater inhibition at lower concentrations than the negatively charged reagents of similar size (Fig. 3C). The positively charged but membrane-permeant MTSEA (35) was very inhibitory with the M57C, A62C, I63C, P64C, and M65C mutants but caused little or no inhibition with the Q52C, F53C, V56C, and V60C mutants.
FIGURE 2.
Reaction of MTS reagents with a cysteine thiolate in a polypeptide chain. MTSEA is membrane-permeable (35). MTS-methyl (MTSM) and the neutral form of MTS-carboxyethyl (MTSCE) are also expected to be membrane-permeable. MTSES and MTSET are membrane-impermeable (35, 37).
FIGURE 3.
Inhibition of ATP-driven proton pumping by MTS reagents. ATP-driven quenching of ACMA fluorescence was measured as described in Fig. 1. The return to maximum fluorescence reached after the addition of nigericin was used to calculate the inhibition values given in Table 2. A, fluorescence quenching of the cG58C mutant with DMSO, 1 mm MTS-methyl (MTSM), 1 mm MTS-carboxyethyl (MTSCE), and 1 mm MTSEA. B, fluorescence quenching of the cG58C mutant with DMSO, 1 mm MTSES, and 1 mm MTSET. C, inhibition of fluorescence quenching of the cG58C mutant by treatment with various concentrations of MTS-methyl (▴), MTS-carboxyethyl (•), MTSEA (▪), MTSES (○), and MTSET (□).
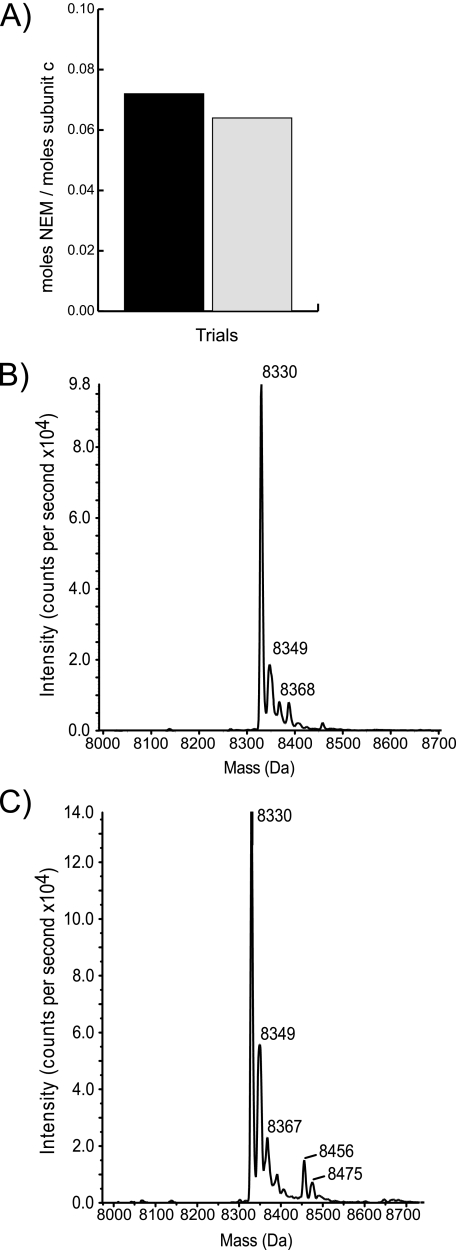
Stoichiometry of [14C]NEM Labeling—The reactivity of cG58C to NEM allowed a determination of the stoichiometry of c subunits modified under conditions approaching maximal inhibition. Radioactive labeling studies were conducted with 0.5 mm [14C]NEM, a concentration that resulted in 90% inhibition of proton pumping versus the maximal inhibition of 94% seen with 5 mm NEM. Subunit c was purified from [14C]NEM-labeled, inverted membrane vesicles, and the incorporation of radioactivity into subunit c was detected using a PhosphorImager (Amersham Biosciences) and also quantitated by scintillation counting. A determination of [14C]NEM incorporation per mg of purified subunit c revealed that the ratio of labeled subunits per total subunits was 0.06–0.07 (Fig. 4A) and suggested that only one monomer per decameric c-ring need react for maximal inhibition.
FIGURE 4.
Stoichiometry of NEM modification of G58C subunit c. A, subunit c was purified from inverted membrane vesicles of the cG58C mutant after treatment with 0.5 mm [14C]NEM for 15 min. The moles of total purified subunit c were determined using a Lowry assay, and moles of incorporated [14C]NEM were determined by scintillation counting. The molar ratio determined in two experiments is indicated. B and C, subunit c was purified from untreated membranes or membrane samples treated with 5 mm NEM for 15 min and then subjected to ESI-TOF mass spectrometry. Primary signal peaks correspond to unlabeled G58C subunit c at 8330 Da and NEM-labeled G58C subunit c at 8456 Da. The peak areas in panel C were used to calculate a ratio of modified to total subunit c equal to 0.12.
The stoichiometry of modification with [14C]NEM is supported by additional experiments using ESI-TOF mass spectrometry. In a sample of purified subunit c from membranes treated with 5 mm NEM, the mass peak corresponding to NEM-modified subunit c was about one-tenth as intense as the mass peak corresponding to unmodified subunit c (Fig. 4, B and C). Unmodified subunit c appeared as a set of peaks with the primary peak at 8330 Da consistent with the calculated mass of subunit c with a G58C substitution. Secondary peaks appeared at 8349 Da (+19 Da) and 8368 Da (+38 Da). The latter corresponds to the adduction of K+ onto subunit c occurring due to the use of K+-containing glassware. The origin of the 19-Da shift is uncertain but is consistent with the adduction of H3O+. In a second experiment G58C mutant membrane vesicles were treated with 5 mm NEM. In the mass spectrum of a sample of purified, NEM-modified subunit c the same set of unmodified peaks was present as well as a second primary peak at 8456 Da, a change in mass consistent with the 126-Da increase in mass predicted for NEM-modified subunit c. The secondary peaks in the NEM-modified peak set were shifted identically by 126 Da relative to the unmodified subunit c. Integration of the area under the peaks sets corresponding to unmodified and modified subunit c revealed a ratio of NEM-modified to unmodified subunit c of 0.12. As shown above, complete inhibition of ATP-driven proton pumping was observed under these labeling conditions and indicated that ∼10% modification is sufficient for total inhibition.
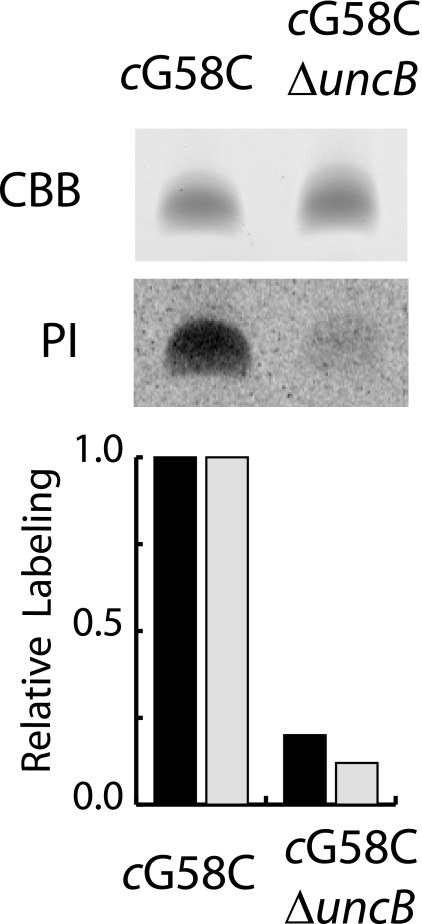
Subunit a Facilitates [14C]NEM Labeling of Subunit c—To determine whether the presence of subunit a is required for aqueous accessibility to this membrane-embedded region of subunit c, we compared the [14C]NEM labeling of subunit c in membranes lacking subunit a to those containing the full F1F0 complex. Subunit c was purified from the labeled vesicles, and incorporated radioactivity was compared using scintillation counting and phosphorimaging analysis. As shown previously, subunit c can insert into the membrane independently of subunit a (38), and the yield of purified subunit c from both sets of membranes was equivalent. The absence of subunit a resulted in an 80% reduction in [14C]NEM labeling of the cG58C residue (Fig. 5).
FIGURE 5.
Labeling of G58C subunit c with [14C]NEM in the presence and absence of subunit a. Inverted membrane vesicles prepared from strains expressing the cG58C mutant and the cG58C ΔuncB mutant were labeled with 0.5 mm [14C]NEM, and subunit c was purified as described under “Experimental Procedures.” 20 μg of purified subunit c solubilized in SDS sample buffer were subjected to SDS-PAGE. The gel was stained with Coomassie Brilliant Blue (CBB), sealed in cellophane, and exposed to a phosphor storage screen for 48 h. Relative labeling was quantitated by scintillation counting (black bars) and from the phosphor image (PI, gray bars).
DISCUSSION
In this study we have substituted Cys for residues on the cytoplasmic side of TMH2 of subunit c and probed the reactivity of these cysteines using thiolate-reactive reagents. We have shown that at least some of these residues are accessible to aqueous solvent from the cytoplasmic side of the membrane and that subunit a facilitates the access of NEM to at least one position in the middle of the membrane.
We were able to test the sensitivity of 10 functional substitutions in the region from residues 52–65. Only the replacement of Leu-59 and the essential Asp-61 resulted in a completely non-functional phenotype. The P64C substitution was constructed in a A20P suppressor background in which it is functional (39). Substitution of Phe-54 and Ile-55 with Cys resulted in a phenotype in which mutants are competent in oxidative phosphorylation as measured by growth on succinate minimal medium but non-functional in the ATP-driven proton pumping assay. Langemeyer and Engelbrecht (40) recently described a similar phenotype for a mutant where the essential Asp-61 residue was relocated to position 65. Because this mutant was also competent in an in vitro ATP synthesis assay, they posit that it represents a class of mutant ATP synthases that can operate in the synthesis direction only.
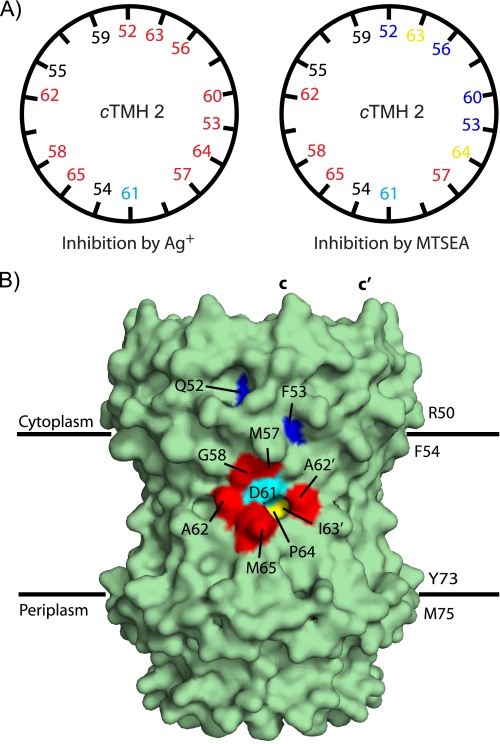
All 10 of the TMH2 substitutions tested were highly sensitive to Ag+. The ionic radius of Ag+ (1.26 Å) is similar to that of H3O+ (1.54 Å) and makes it small enough to reach side chains in aqueous accessible environments. The extent of aqueous accessibility in cTMH2 is surprising given the hydrophobicity of the region. Residues on all faces of the α-helix proved to be sensitive to Ag+, a result which suggests that there may be some helical mobility in this region (Fig. 6A). If H2O and Ag+ only have access to residues packed at the subunit a-c interface, then reaction of Ag+ with residues on the helical face opposite to Asp-61 would require a turning of cTMH2. Cross-linking experiments support the idea of helical mobility in this region (41).
FIGURE 6.
Mapping of reactive residues on structural models of subunit c. A, residues on the cytoplasmic side of cTMH2 are represented in an α-helical wheel model. Red coloring indicates reactivity to Ag+ (>90% inhibition) or MTSEA (>80% inhibition). Yellow-colored positions were moderately sensitive, and blue-colored positions were insensitive to MTSEA treatment. Black residues could not be tested. Asp-61 is colored cyan. B, this model of the E. coli c-ring was derived by Meier et al. (12) from the x-ray structure of the c-ring from I. tartaricus using the program WHATIF to substitute E. coli residues. The positions of MTSEA-sensitive residues appear as colored patches on the surface of the ring. Coloring indicates Asp-61 (cyan), residues highly sensitive to MTSEA (red), and residues moderately sensitive to MTSEA (yellow). The areas of residues Phe-53 and Gln-52 exposed to the surface are colored blue. The side chains of residues Val-56 and Val-60 lie behind the surface shown. The surface shown has been calculated using the wild type side chains.
In contrast to the sensitivity to Ag+, the sensitivity of the substituted cysteines to the larger probes was more limited. Mapping of the NEM- and MTS-sensitive positions on a structural model of the c-ring reveals a cluster of sensitive residues in the middle of the membrane surrounding the essential aspartate at position 61 (Fig. 6B). The most sensitive residue in this pocket was Gly-58, which was inhibited significantly by all the thiolate-reactive reagents tested. Gly-58 lies on the same face of TMH2 as Asp-61, one helical turn to the cytoplasmic side. Based on cross-linking studies describing the interaction of cTMH2 and aTMH4 (42), chemical modifications of G58C could sterically and/or electrostatically interrupt the interaction between Asp-61 and the essential Arg-210 on aTMH4. This interference may also occur upon modification of M57C by the larger MTS reagents, MTSES and MTSET. All of the residues in the reactive pocket are especially sensitive to inhibition by the small, positively charged reagent MTSEA. The introduction of the positively charged amine in the vicinity of Asp-61 may lower the pKa of the Asp-61, making it ill-suited for proton binding. Other small MTS reagents like MTS-methyl may also react with the MTSEA-sensitive residues but not inhibit proton translocation. Functional inhibition is the only method by which we can currently measure reaction with MTS reagents. Significant sensitivity to the larger, membrane-impermeable MTS reagents, MTSES and MTSET, was restricted to residues on the cytoplasmic side of Asp-61. The inability of the positively charged MTSET to inhibit MTSEA-sensitive residues on the periplasmic side of Asp-61 suggests that MTSET cannot access these sites from the cytoplasm. The pattern of MTSEA inhibition is generally consistent with a structural model of the c-ring based on the x-ray structure of the c11-ring of I. tartaricus. Residues exposed on the periphery of the ring are most sensitive to treatment with MTSEA, whereas residues buried between TMH2 and TMH1 are less sensitive or unaffected, e.g. Glu-52, Phe-53, Val-56, Val-60, and Ile-63 (Fig. 6A).
We have determined that only one c monomer per decameric ring is modified by NEM and that this single modification is sufficient to cause complete inhibition of proton translocation. Maximal inhibition by dicyclohexylcarbodiimide is observed with similar substoichiometric modification (29). The low stoichiometry of modification leading to maximal inhibition is consistent with a model in which subunit a provides aqueous access to subunit c, since only one or perhaps two c subunits can interact with subunit a at any given time. The most likely location for the modified c subunit is at the a-c interface. In a model where each c subunit has intrinsic access to the cytoplasm (3), a higher modification stoichiometry would be expected.
In support of the contribution of subunit a to the cytoplasmic exit channel, we have determined that access by NEM to the membrane-embedded region surrounding Asp-61 is dependent on the presence of subunit a. The simplest interpretation of the dependence is that subunit a contributes to an aqueous pathway from the middle of the membrane to the cytoplasm. This interpretation is supported by the mapping of Ag+-sensitive residues to the cytoplasmic side of TMH4 in subunit a (20). The Ag+ accessibility to the cytoplasmic domains of both cTMH2 and aTMH4 suggests that the aqueous pathway likely exists at the subunit a-c interface. Further studies are now required to expand the aqueous accessibility map of subunit c and better define the contribution of subunit a to aqueous accessibility in subunit c.
Acknowledgments
We thank Jessica Carrano for the preparation of some of the cysteine substitution mutants used in this study and Dr. Gregory Barrett-Wilt and Dr. Amy Harms for assistance with the mass spectrometry experiments. Mass spectrometry data were collected at the Mass Spectrometry Facility at the University of Wisconsin Biotechnology Center.
This work was supported by United States Public Health Service, National Institutes of Health Grant GM23105. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TMH, transmembrane helix; ACMA, 9-amino-6-chloro-2-methoxyacridine; ESI-TOF, electrospray ionization time of flight; NEM, N-ethylmaleimide; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine; MTS, methanethiosulfonate; MTSEA, MTS-ethylamino; MTSES, MTS-ethylsulfonate; MTSET, MTS-ethyl(trimethylammonium).
References
- 1.Yoshida, M., Muneyuki, E., and Hisabori, T. (2001) Nat. Rev. Mol. Cell Biol. 2 669-677 [DOI] [PubMed] [Google Scholar]
- 2.Capaldi, R. A., and Aggeler, R. (2002) Trends Biochem. Sci. 27 154-160 [DOI] [PubMed] [Google Scholar]
- 3.Dimroth, P., von Ballmoos, C., and Meier, T. (2006) EMBO Rep. 7 276-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber, J., and Senior, A. (2003) FEBS Lett. 545 61-70 [DOI] [PubMed] [Google Scholar]
- 5.Jiang, W., Hermolin, J., and Fillingame, R. H. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4966-4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stock, D., Leslie, A. G., and Walker, J. E. (1999) Science 286 1700-1705 [DOI] [PubMed] [Google Scholar]
- 7.Meier, T., Yu, J., Raschle, T., Henzen, F., Dimroth, P., and Muller, D. J. (2005) FEBS J. 272 5474-5483 [DOI] [PubMed] [Google Scholar]
- 8.Pogoryelov, D., Yu, J., Meier, T., Vonck, J., Dimroth, P., and Muller, D. J. (2005) EMBO Rep. 6 1040-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noji, H., Yasuda, R., Yoshida, M., and Kinosita, K., Jr. (1997) Nature 386 299-302 [DOI] [PubMed] [Google Scholar]
- 10.Abrahams, J. P., Leslie, A. G., Lutter, R., and Walker, J. E. (1994) Nature 370 621-628 [DOI] [PubMed] [Google Scholar]
- 11.Boyer, P. D. (1989) FASEB J. 3 2164-2178 [DOI] [PubMed] [Google Scholar]
- 12.Jones, P. C., Jiang, W., and Fillingame, R. H. (1998) J. Biol. Chem. 273 17178-17185 [DOI] [PubMed] [Google Scholar]
- 13.Meier, T., Polzer, P., Diederichs, K., Welte, W., and Dimroth, P. (2005) Science 308 659-662 [DOI] [PubMed] [Google Scholar]
- 14.Valiyaveetil, F. I., and Fillingame, R. H. (1998) J. Biol. Chem. 273 16241-16247 [DOI] [PubMed] [Google Scholar]
- 15.Schwem, B. E., and Fillingame, R. H. (2006) J. Biol. Chem. 281 37861-37867 [DOI] [PubMed] [Google Scholar]
- 16.Fillingame, R. H., Angevine, C. M., and Dmitriev, O. Y. (2003) FEBS Lett. 555 29-34 [DOI] [PubMed] [Google Scholar]
- 17.Karlin, A., and Akabas, M. H. (1998) Methods Enzymol. 293 123-145 [DOI] [PubMed] [Google Scholar]
- 18.Kaback, H. R., Dunten, R., Frillingos, S., Venkatesan, P., Kwaw, I., Zhang, W., and Ermolova, N. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 491-494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu, Q., and Miller, C. (1995) Science 268 304-307 [DOI] [PubMed] [Google Scholar]
- 20.Angevine, C. M., and Fillingame, R. H. (2003) J. Biol. Chem. 278 6066-6074 [DOI] [PubMed] [Google Scholar]
- 21.Angevine, C. M., Herold, K. A., and Fillingame, R. H. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 13179-13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angevine, C. M., Herold, K. A., Vincent, O. D., and Fillingame, R. H. (2007) J. Biol. Chem. 282 9001-9007 [DOI] [PubMed] [Google Scholar]
- 23.von Ballmoos, C., Meier, T., and Dimroth, P. (2002) Eur. J. Biochem. 269 5581-5589 [DOI] [PubMed] [Google Scholar]
- 24.Meier, T., Matthey, U., von Ballmoos, C., Vonck, J., Krug von Nidda, T., Kuhlbrandt, W., and Dimroth, P. (2003) J. Mol. Biol. 325 389-397 [DOI] [PubMed] [Google Scholar]
- 25.Barik, S. (1996) Methods Mol. Biol. 57 203-215 [DOI] [PubMed] [Google Scholar]
- 26.Valiyaveetil, F. I., and Fillingame, R. H. (1997) J. Biol. Chem. 272 32635-32641 [DOI] [PubMed] [Google Scholar]
- 27.Fillingame, R. H. (1975) J. Bacteriol. 124 870-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deleted in proof
- 29.Hermolin, J., and Fillingame, R. H. (1989) J. Biol. Chem. 264 3896-3903 [PubMed] [Google Scholar]
- 30.Fillingame, R. H. (1976) J. Biol. Chem. 251 6630-6637 [PubMed] [Google Scholar]
- 31.Schaegger, H., and von Jagow, G. (1987) Anal. Biochem. 166 368-379 [DOI] [PubMed] [Google Scholar]
- 32.Bell, R., and Kramer, J. (1999) Environ. Toxicol. Chem. 18 9-22 [Google Scholar]
- 33.Friedman, M. (1973) The Chemistry and Biochemistry of the Sulfhydryl Group in Amino Acids, Peptides, and Proteins, Oxford, Pergemon Press
- 34.Roberts, D. D., Lewis, S. D., Ballou, D. P., Olson, S. T., and Shafer, J. A. (1986) Biochemistry 25 5595-5601 [DOI] [PubMed] [Google Scholar]
- 35.Holmgren, M., Liu, Y., Xu, Y., and Yellen, G. (1996) Neuropharmacology 35 797-804 [DOI] [PubMed] [Google Scholar]
- 36.Liu, Y., Jurman, M. E., and Yellen, G. (1996) Neuron 16 859-867 [DOI] [PubMed] [Google Scholar]
- 37.Kwaw, I., Zen, K. C., Hu, Y., and Kaback, H. R. (2001) Biochemistry 40 10491-10499 [DOI] [PubMed] [Google Scholar]
- 38.Hermolin, J., and Fillingame, R. H. (1995) J. Biol. Chem. 270 2815-2817 [DOI] [PubMed] [Google Scholar]
- 39.Fimmel, A. L., Jans, D. A., Langman, L., James, L. B., Ash, G. R., Downie, J. A., Senior, A. E., Gibson, F., and Cox, G. B. (1983) Biochem. J. 213 451-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langemeyer, L., and Engelbrecht, S. (2007) Biochim. Biophys. Acta 1767 998-1005 [DOI] [PubMed] [Google Scholar]
- 41.Vincent, O. D., Schwem, B. E., Steed, P. R., Jiang, W., and Fillingame, R. H. (2007) J. Biol. Chem. 282 33788-33794 [DOI] [PubMed] [Google Scholar]
- 42.Jiang, W., and Fillingame, R. H. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 6607-6612 [DOI] [PMC free article] [PubMed] [Google Scholar]