Abstract
Apolipoprotein AII (apoAII) transgenic (apoAIItg) mice exhibit several traits associated with the insulin resistance (IR) syndrome, including IR, obesity, and a marked hypertriglyceridemia. Because treatment of the apoAIItg mice with rosiglitazone ameliorated the IR and hypertriglyceridemia, we hypothesized that the hypertriglyceridemia was due largely to overproduction of very low density lipoprotein (VLDL) by the liver, a normal response to chronically elevated insulin and glucose. We now report in vivo and in vitro studies that indicate that hepatic fatty acid oxidation was reduced and lipogenesis increased, resulting in a 25% increase in triglyceride secretion in the apoAIItg mice. In addition, we observed that hydrolysis of triglycerides from both chylomicrons and VLDL was significantly reduced in the apoAIItg mice, further contributing to the hypertriglyceridemia. This is a direct, acute effect, because when mouse apoAII was injected into mice, plasma triglyceride concentrations were significantly increased within 4 h. VLDL from both control and apoAIItg mice contained significant amounts of apoAII, with ∼4 times more apoAII on apoAIItg VLDL. ApoAII was shown to transfer spontaneously from high density lipoprotein (HDL) to VLDL in vitro, resulting in VLDL that was a poorer substrate for hydrolysis by lipoprotein lipase. These results indicate that one function of apoAII is to regulate the metabolism of triglyceride-rich lipoproteins, with HDL serving as a plasma reservoir of apoAII that is transferred to the triglyceride-rich lipoproteins in much the same way as VLDL and chylomicrons acquire most of their apoCs from HDL.
More than 95% of plasma apolipoprotein AII (apoAII)2 is associated with high density lipoproteins (HDLs) where it is the second most abundant protein comprising ∼20% of the HDL total protein mass (1). Studies of the physiologic effects of apoAII have focused on reverse cholesterol transport and anti-oxidant functions, mechanisms through which HDLs are believed to protect against atherosclerosis (2, 3). Most but not all of these studies suggest that increased apoAII impaired both of these processes and promoted atherosclerosis (4-8). Additional evidence for other physiologic effects of apoAII has come from genetic studies in both humans and mice, which suggest a role for apoAII in triglyceride and free fatty acid metabolism (8-10). Subsequently, studies of genetically modified mice carrying a transgene for either human or mouse apoAII (3, 11-21), as well as apoAII knockout mice (22), confirmed a complex metabolic role for apoAII, including a rather profound effect on plasma triglyceride concentrations.
Hypertriglyceridemia is an integral component of the phenotype of several disease states, including type 2 diabetes, familial combined hyperlipidemia, and the metabolic syndrome, and it is likely to be one of several contributing factors underlying the increased atherosclerosis associated with these complex diseases. Hypertriglyceridemia has been demonstrated to be an independent risk factor for the development of premature atherosclerosis, although the mechanisms involved are unclear (23, 24). It is known that larger, more triglyceride-rich VLDL (VLDL1) are the precursors for the more atherogenic small dense LDL (25). Furthermore, intermediate density lipoproteins, or remnant lipoprotein particles, which also derive from the catabolism of VLDL, are also pro-atherogenic (26). Hypertriglyceridemia also increases the availability of plasma free fatty acids (FFAs), which can dramatically alter metabolism in several tissues such as skeletal muscle and adipose, inducing additional metabolic changes such as obesity and insulin resistance. It is noteworthy that the plasma levels of FFA are increased under conditions in which apoAII is elevated, both in mice and humans (9).
While type 2 diabetes is clearly associated with hypertriglyceridemia, the mechanisms underlying this association are not clear. Hypertriglyceridemia can increase the availability of fatty acids to various tissues, primarily skeletal muscle and adipose, after hydrolysis by lipoprotein lipase. Mice that have a muscle-specific overexpression of lipoprotein lipase develop skeletal muscle insulin resistance as a consequence of increased fatty acids taken up from plasma triglycerides (27). Thus, insulin resistance can occur as a result of hypertriglyceridemia. On the other hand, chronic increases in plasma insulin concentrations and fatty acids, as would occur in obesity and type 2 diabetes, have been demonstrated to cause hypertriglyceridemia through increased production of VLDL by the liver (28-33). In this scheme, which currently enjoys widespread acceptance with respect to the etiology of hypertriglyceridemia associated with type 2 diabetes, increased FFA released from the adipose tissue are cleared by the liver, and due to chronic exposure to insulin, VLDL synthesis is increased.
We previously demonstrated that mice, which overexpress mouse apoA-II, exhibit a marked hypertriglyceridemia, hypercholesterolemia, and increased plasma FFA, as well as insulin resistance, increased adiposity, and increased atherosclerosis (3, 5, 6, 20, 21). Treatment of the apoAIItg mice with an insulinsensitizing PPARγ agonist ameliorated the insulin resistance and hypertriglyceridemia (20). This suggested that the hypertriglyceridemia was mostly a consequence of the insulin resistance, consistent with the hypothesis described above. In their early development, PPARγ agonists were believed to act primarily by reducing insulin resistance in adipose tissue. However, it is now known that PPARγ agonists also have additional direct effects on other tissues, including liver and skeletal muscle (34, 35). Therefore, the possibility that treatment with PPARγ agonists reduced hypertriglyceridemia first and that insulin resistance was ameliorated as a consequence could not be ruled out.
ApoAII levels are associated with plasma concentrations of free fatty acids and triglycerides in humans, as well as with type 2 diabetes, atherosclerosis, and abdominal obesity (10, 36-39, 41-47). ApoAII is also involved in the hypertriglyceridemia of familial combined hyperlipidemia (48), possibly as a target gene of the transcription factor, USF-1 (49), which has been implicated in the development of the familial combined hyperlipidemia phenotype (48, 50) and is known to regulate the expression of several genes involved in glucose and fatty acid metabolism. Understanding the mechanisms through which apoAII increases plasma triglycerides is thus relevant to the understanding of several important human diseases.
In the present study we demonstrate that, although overproduction of triglycerides by the liver contributes to the hypertriglyceridemia in the apoAIItg mice, the primary underlying cause is a decrease in the rates of both VLDL and chylomicron clearance. This decreased clearance does not appear to be due to changes in lipase activity in the apoAIItg mice but rather to the fact that elevated apoAII makes the lipoproteins a poorer substrate for lipolytic activity. Administration of mouse apoAII produced a rapid increase in plasma VLDL triglyceride concentrations in vivo. This effect was consistent with in vitro experiments where apoAII was transferred from HDL to VLDL, which subsequently was a poorer substrate for hydrolysis by lipoprotein lipase. We conclude that the effects of mouse apoAII overexpression on hypertriglyceridemia appear to represent an acute response to increased plasma apoAII concentrations, as well as a chronic indirect effect that requires the development of insulin resistance. Although the hypertriglyceridemia and increased FFA may contribute to the IR syndrome in this model, our results suggest that factors other than the increased plasma FFA and triglycerides may initiate the skeletal muscle insulin resistance.
EXPERIMENTAL PROCEDURES
Animals—Transgenic mice containing multiple copies of the mouse apoAII gene on a C57BL/6J background, were produced as described previously (5, 6). All animals were housed 3-4 to a cage, maintained at 24 °C on a 12-h light-dark cycle, and provided Harlan-Teklad rodent chow (6% fat) and water ad libitum. The care of the mice, as well as all procedures used in this study, was done in accordance with NIH animal care guidelines. ApoAIItg animals used in the present study were males homozygous for the apoAII transgene, expressing plasma apoAII concentrations that were on average 5-fold higher than the age matched male C57BL/6J mice that were used as the controls. The apoAII transgene was also bred onto an apoEko background to investigate potential interactions of apoAII and apoE in the development of hypertriglyceridemia.
Lipid Analyses—Plasma was collected from mice that were fasted overnight and bled 2-3 h after the beginning of the light cycle from the retro-orbital plexus under isoflorane anesthesia. Total cholesterol, HDL cholesterol, triglycerides, and FFA concentrations were determined in triplicate as described previously (6, 51). HDL was isolated by precipitation of VLDL and LDL with heparin and manganese chloride (51). An external control sample with known analyte concentration was run in each plate to ensure accuracy. The lipid composition of ultracentrifugally isolated VLDLs was also determined by the lipid assays described above, as well as analysis of their phospholipid content (phospholipid C kit #433-36201, Wako Chemicals, Richmond, VA). Our laboratory participates in the Centers for Disease Control Lipid Standardization program (Laboratory ID# LSP-251), undergoing quarterly certification by the Centers for Disease Control.
In Vivo Rates of VLDL Secretion—Rates of in vivo VLDL secretion were determined using tyloxapol (Triton WR-1339) to inhibit VLDL clearance from the plasma, as described previously (52). Briefly, following an overnight fast a 15% solution of tyloxapol in 0.9% NaCl was injected (100 mg/kg body wt) via the tail vein. Immediately following administration of tyloxapol, a 50-μl blood sample was drawn from the retro-orbital plexus, and subsequent plasma samples were obtained at the 30-min and 1-h time points. Triglyceride concentrations were determined in plasma samples as described above.
Hepatic Fatty Acid Oxidation in Liver Sections—Following an overnight fast, mice were anesthetized with isoflorane and sacrificed by cervical dislocation. The left caudal lobe of the liver was rapidly excised, and fresh liver sections of uniform thickness (averaging ∼0.06 g) were obtained using a Stadie-Riggs microtome. The liver sections were weighed and immediately incubated at 37 °C for 40 min in Krebs-Henseleit buffer under 95% O2:5% CO2, that contained 5.5 mm glucose, 3% bovine serum albumin, 1 mm oleate, and [U-14C]palmitic acid (2.5 μCi/ml). The average time from sacrificing the animals to getting the liver section into the buffer was <2 min. Rates of FA oxidation were assessed by determining 14CO2 trapped in hyamine hydroxide-saturated filter paper as described previously (53).
Triglyceride Secretion from Liver Sections—Liver sections were obtained and incubated essentially as described above for determining rates of fatty acid oxidation, except that media were collected following a 2-h incubation period. Total lipids were extracted using the method of Folch et al. (54). Following extraction, the lipid classes were separated by TLC, and the radioactivity in the triglyceride fraction was determined by liquid scintillation spectrometry (55). Radioactivity in media triglycerides represents VLDL secretion.
Hepatic Lipogenesis—Liver sections were obtained and incubated essentially as described above, except that 3H2O (0.5 mCi/vessel) was used as the radioactive tracer and incubations were carried out for 1 h (56). Media and tissue were then collected and total lipids extracted as described above. Total lipid isolates were then hydrolyzed in an acid/acetonitrile solution as described previously, and the lipids were re-extracted and dried under nitrogen (55). The sample was then separated by TLC and radioactivity in the fatty acid fraction determined by liquid scintillation spectrometry (55).
Acute Effects of ApoAII on VLDLs in Vivo—Apolipoproteins AI and AII were isolated from delipidated apoAIItg mouse HDL using a Sephacryl 200 column as described previously (57). The HDL from the apoAIItg mice contained approximately equal amounts of apoAI and apoAII, which eluted as two separate peaks (57). The protein composition of the individual fractions of apoAI and apoAII were determined by SDS-PAGE followed by silver staining and Western blotting. Fractions of apoAI and apoAII, which were at least 96% pure were then dialyzed against PBS containing dimyristoylphosphatidylcholine to aid in solubility as the urea was removed. Following dialysis the protein content of each preparation was determined. 4 mg of either the apoAI or apoAII was then administered via tail vein injection to C57BL/6J control mice that had been fasted overnight. Mice were bled from the retro-orbital plexus under isoflorane anesthesia just prior to injection and at 4 h post-injection. Plasma triglycerides, glucose, and insulin concentrations were then determined. The remaining plasma samples for each respective group (apoAI-injected and apoAII-injected) were then pooled, the VLDL was isolated by ultracentrifugation, and the protein content was determined by Western blot analysis as described below.
Chylomicron/Retinyl Palmitate Clearance Studies—Mice were fasted for 4 h, beginning at 5:00 a.m. Beginning at 9:00 a.m., 5000 IU of retinyl palmitate (all-trans) or vehicle alone (corn oil) was administered by oral gavage (100 μl). A zero time bleed was obtained from the retro-orbital plexus just prior to administration of the retinyl palmitate, and subsequent bleeds followed at 1, 2, 4, and 10 h post gavage. Plasma concentrations of triglycerides were determined as described above. Plasma levels of retinyl esters were measured by high-performance liquid chromatography on a 250 × 4.6 mm Beckman Ultrasphere C18 column (58, 59). Retinyl esters were separated in a mobile phase consisting of acetonitrile:methanol:dichloromethane (70:15:15, v/v) at a flow rate of 1.8 ml/min and detected by UV absorbance at 325 nm. Retinyl acetate was used as an internal standard. Retinyl ester standards were synthesized from authentic all-trans-retinol and the corresponding fatty acyl chloride (60). The reported plasma retinyl ester concentrations represent the sum of individual retinyl ester concentrations (retinyl linoleate, retinyl oleate, retinyl palmitate, and retinyl stearate). All extraction and high-performance liquid chromatography procedures were carried out under N2 and reduced light to prevent oxidation of the compounds. Plasma samples (25-200 μl) were denatured with an equal volume of absolute ethanol containing known amounts of retinyl acetate and then extracted into hexane. Following phase separation, the hexane extract was evaporated under a stream of N2, and the residue was resuspended in benzene for injection onto the high-performance liquid chromatography column. The lower limit of detection for retinyl esters was 2 ng/ml (61).
In Vitro Rates of VLDL Hydrolysis—VLDL (d < 1.0063) were isolated from plasma of overnight fasted control and apoAIItg mice by ultracentrifugation as described previously (3). The VLDL were then washed and concentrated using a centricon-10 micro-concentrator, and VLDL total protein and triglyceride concentrations were determined. VLDL at a final triglyceride concentration of 90 mg/dl was then incubated with isolated lipoprotein lipase (LPL) (Sigma bovine milk LPL) at a final concentration of 24 μg/ml. Total volume in each reaction was 50 μl, and 4 tubes for each time point were incubated at 37 °C for 20, 40, 60, 120, and 180 min. At the end of each incubation period, the hydrolysis was stopped by adding 17 μl of 8 m urea to the reaction (2 m final urea concentration) and placing the tube on ice. The fatty acid concentration in duplicate aliquots from each tube was then determined as described above. The initial fatty acid concentration (0 time) was determined by setting up the reaction and immediately adding the urea without incubating at 37 °C.
Protein Determinations—Total protein content was assayed in triplicate determinations using the Bio-Rad DC protein assay (cat# 500-0116) with bovine serum albumin standards (Bio Rad protein assay standard II, cat #500-0007).
In Vitro Effects of HDL on VLDL Hydrolysis—VLDL (d < 1.0063) and HDL (d = 1.063-1.21) were isolated from fasted C57BL/6J and apoAIItg mice. Both types of VLDL were then separately incubated with either PBS alone in a final volume of 500 μl, or with PBS containing HDL of the same strain, or HDL of the alternate strain. The lipoprotein concentrations in the incubations were 0.34 mg of VLDL protein/ml (with or without HDL) and 2.0 mg of HDL protein/ml. The lipoproteins were incubated with gentle shaking at 37 °C for 1 h. After the incubation period 400 μl of each incubate was applied to an fast-protein liquid chromatography system, and the VLDL and HDL fractions were re-isolated by gel filtration chromatography using two Superose 6 columns connected in series. Fractions 21-25 containing the VLDL, and fractions 54-58 containing the HDL, were pooled separately and concentrated using a Centricon centrifugal concentrator with a molecular mass cutoff of 3.5 kDa (Ambion Inc.). Protein assays were performed, and the recovery of VLDL protein in the sample that was not incubated with HDL was ∼82%. The recovery of VLDL protein in the sample that was incubated with HDL was 110%. These preliminary protein recovery results suggest that the protein content of the VLDL increased ∼30% after incubation with HDL. Western blot analysis was performed, as described below, to determine the content of specific apolipoproteins, apoAI, apoAII, apoAIV, apoCI, apoCII, apoCIII, and apoE. Aliquots of the VLDL re-isolated after in vitro incubation with HDL were then tested in the hydrolysis assay as described above.
Transfer of Apolipoproteins from HDL to VLDL through Dialysis Tubing—Ultracentrifugally isolated VLDL (d < 1.0063) from C57BL/6J control mice in PBS were placed in dialysis tubing (50-kDa molecular mass cutoff) and dialyzed against either PBS alone, or PBS containing ultracentrifugally isolated HDL (d = 1.063-1.21) from apoAIItg mice. An additional control was performed with only PBS inside the dialysis tubing and PBS with apoAII HDL on the outside. After incubation for 12 h with gentle agitation on a rotary shaker at room temperature, the contents of the dialysis tubing (VLDL dialyzed against PBS, VLDL dialyzed against HDL, and PBS dialyzed against HDL) were recovered, protein content was determined, and equal amounts of VLDL protein were subjected to Western blot analysis for apoAII and apoCII. No protein was detectable in the PBS that was dialyzed against HDL, however, this sample was still concentrated using an Amicon centrifugal concentrator (molecular mass cutoff, 3.5 kDa) and also subjected to Western blot analysis.
Western Blot Analysis of Apolipoproteins—Relative amounts of apolipoproteins apoAI, apoAII, apoAIV, apoCI, apoCII, apo-CIII, apoE, and apoB in VLDL were determined by a combination of SDS-PAGE and immunoblotting. Sample loading was normalized by loading the same amount of total protein in each lane. Two sets of molecular weight markers were also applied to each gel (Rainbow RPN800, GE Healthcare Bio-Sciences Corp, Piscataway, NJ, and MagicMark XP, Invitrogen). The samples were electrophoresed on 4-20% Tris/glycine polyacrylamide gradient gels (Novex) under reducing, denaturing conditions. Samples were diluted in sample buffer (10% 2-mercaptoethanol, 0.25 m Tris-HCl, pH 6.8, 0.2% SDS, 20% glycerol, and 0.025% bromthymol blue), and 17 μl of each sample preparation containing 4 μg of total protein was loaded. Following electrophoreses, the proteins were transferred to nitrocellulose using a wet blotter, probed with anti-mouse antibodies from Biodesign International (Camarillo, CA) diluted 1:2000 in 5% milk-PBST (phosphate buffered saline with Tween) and quantitated by chemiluminescent detection (Amersham Biosciences).
Plasma Concentrations of TNF-α and MCP-1—Concentrations of TNF-α and MCP-1 were determined in plasmas of control and apoAIItg mice that had been maintained on a standard low fat chow diet and that had been fasted overnight. Assays were run in duplicate determinations using commercially available enzyme-linked immunosorbent assay kits for both TNF-α and MCP-1 (Mouse TNF-α EIA, catalog #45TNFMS-E01 and mouse MCP-1 EIA, catalog #45-MCPMS-E01, Alpco Diagnostics, Salem, NH).
RESULTS
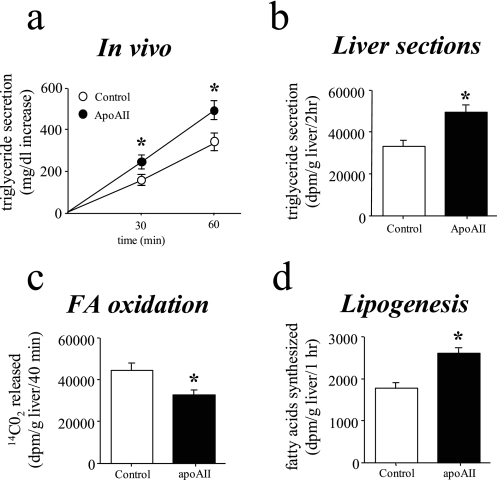
Elevated ApoAII Expression Increases Triglyceride Secretion—In earlier experiments we observed that fasting glucose concentrations were increased 20-40% in the male apoAIItg mice and that fasting insulin concentrations were 3-fold higher than controls (20, 21). Furthermore, other findings, such as a 2-fold increase in hepatic glycogen, were consistent with the liver in the apoAIItg mice responding normally to the chronically increased plasma insulin and glucose concentrations (20, 21). Therefore, it was likely that increased secretion of VLDL was contributing to the hypertriglyceridemia in the apoAIItg mice. In vivo rates of VLDL secretion were determined in fasted animals using tyloxapol (Triton wr-1339) to block lipolysis of triglyceride-rich lipoproteins. Compared with control mice, VLDL secretion was increased ∼20% in the apoAIItg mice (Fig. 1a). Livers from control and apoAIItg mice that had been fasted overnight had similar total wet weights (1.3 ± 0.1 and 1.4 ± 0.1 g for control and apoAIItg, respectively), as well as similar concentrations of triglycerides (10.4 ± 1.5 and 7.6 ± 0.8 mg/g liver) and total cholesterol (0.49 ± 0.04 and 0.43 ± 0.07 mg/g liver).
FIGURE 1.
Rates of triglyceride secretion, hepatic fatty acid oxidation, and lipogenesis. a, rates of in vivo triglyceride secretion were determined in apoAIItg and control mice that had been fasted overnight. Triton WR-1339 (100 mg/kg body wt) was administered via the tail vein. The plasma triglyceride concentrations were determined at the initial “0 time” bleed and at 30 min and 1 h post injection. Triglyceride secretion was calculated as the increase in total plasma triglyceride concentration over the 0 time values, which were 56 ± 4 and 287 ± 32 mg/dl for control and apoAIItg mice, respectively. Data represent the mean ± S.E. for eight animals in each group. *, values significantly different (p < 0.05) than control mice. b, rates of triglyceride secretion were determined in liversections obtained from apoAIItg and control mice that had been fasted overnight. The liver sections were weighed and immediately incubated for 2 h in Krebs-Henseleit buffer that contained a bovine serum albumin/[U-14C]palmitic acid (2.5 μCi/ml) complex. Total lipids were extracted from the media and separated into various lipid fractions by TLC. The radioactivity in the triglyceride fraction was determined by liquid scintillation spectrometry. Data represent the mean ± S.E. for eight animals in each group. *, values significantly different (p < 0.05) than control mice. c, oxidation of 14C-labeled palmitate to 14CO2 was determined in liver sections obtained from control and apoAIItg mice that had been fasted overnight. Data represent the mean ± S.E. for six animals in each group. *, values that are significantly different (p < 0.05) than control mice. d, rates of lipogenesis were determined in liver sections obtained from mice that had been fasted overnight. They were incubated for 1 h in Krebs-Henseleit bicarbonate buffer containing 3H2O (0.5 mCi/vessel) as the radioactive tracer to label newly synthesized fatty acids. Total lipid extract of tissue and media were then hydrolyzed, and the lipids were re-extracted and dried under nitrogen. The sample was then separated by TLC, and radioactivity in the fatty acids was determined by liquid scintillation spectrometry. Data represent the mean ± S.E. for six animals in each group. *, values that are significantly different (p < 0.05) than control mice.
Plasma fatty acid concentrations were significantly increased in the apoAIItg mice compared with controls (5, 6, 20, 21). To determine if this increased supply of fatty acids was responsible for the increased triglyceride secretion, triglyceride secretion rates also were determined in vitro using liver sections obtained from animals that had been fasted overnight. Consistent with the results obtained from the in vivo studies, even when supplied with the same amount of exogenous fatty acids, hepatic secretion of VLDL was increased ∼25% in liver sections obtained from the apoAIItg mice (Fig. 1b).
The increase in triglyceride secretion could also result from reduced oxidation of fatty acids, thereby directing the available fatty acid supply into the pathways of esterification. To address this possibility, rates of fatty acid oxidation to CO2 were determined in liver sections from animals that had been fasted overnight. Rates of fatty acid oxidation were reduced ∼22% in liver sections from the apoAIItg mice (Fig. 1c).
Increased rates of hepatic de novo fatty acid synthesis (lipogenesis) could also supply additional fatty acids for the increased triglyceride secretion. Rates of lipogenesis were determined in liver sections from animals that had been fasted overnight. Compared with the control mice, rates of lipogenesis were increased ∼20% in liver sections from the apoAIItg mice (Fig. 1d), consistent with both the decreased rates of oxidation and increased secretion of triglycerides.
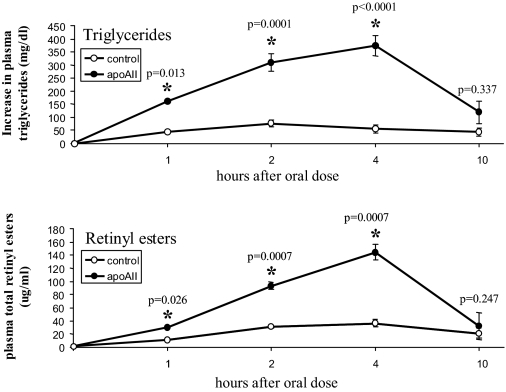
Elevated Plasma ApoAII Decreases Turnover and Lipolysis of Triglyceride-rich Lipoproteins—The effects we observed in the in vivo and in vitro experiments were consistent with a normal response of liver to chronically elevated plasma insulin concentrations, with increased rates of lipogenesis, decreased rates of fatty acid oxidation and increased triglyceride secretion (29-33). However, because the plasma triglyceride concentrations in the apoAIItg mice were elevated ∼5-10 fold compared with the control mice, it did not appear likely that a ∼25% increase in triglyceride secretion would result in a severalfold increase in plasma triglyceride concentrations. Therefore, we also determined in vivo clearance rates of chylomicrons following an oral gavage of retinyl palmitate/corn oil. Compared with the control mice, chylomicron clearance rates were significantly delayed in the apoAIItg mice (Fig. 2), indicating that decreased clearance was a major factor contributing to the hypertriglyceridemia.
FIGURE 2.
Chylomicron clearance. Mice were fasted for 4 h, beginning at 5:00 a.m. Beginning at 9:00 a.m. retinyl palmitate/corn oil was administered by oral gavage (150 μl per animal). A zero time bleed was obtained from the retro-orbital plexus just prior to administration of the retinyl palmitate, and subsequent bleeds followed at 1, 2, 4, and 10 h post gavage. Triglyceride (upper panel) and total retinyl ester (lower panel) plasma concentrations were then determined at each time point. Data represent the mean ± S.E. for eight animals in each group for the changes in triglycerides and retinyl esters from the 0 time values. *, values that are significantly different than control mice.
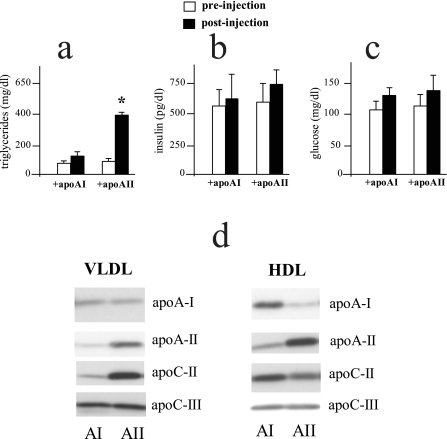
To further examine whether the primary mechanism contributing to the hypertriglyceridemia was a chronic effect of apoAII overexpression we asked whether acutely increasing apoAII by intravenous injection could alter plasma triglyceride concentrations. When equal amounts of mouse apoAII or mouse apoAI were injected into control mice and plasma lipids determined 4 h later, those that received apoAII exhibited an ∼3-fold increase in plasma triglycerides compared with mice that received apoAI (Fig. 3a). Although triglyceride concentrations were significantly increased by apoAII, no differences in plasma FFA concentrations were observed after 4 h (44 ± 4 and 47 ± 6 mg/dl). These experiments were performed on two separate occasions with similar results. In the second series of experiments we also determined plasma insulin and glucose concentrations 4 h after administration of either apoAI or apoAII as well as the changes in apoAI, apoAII, apoCII, and apoCIII in the respective VLDL and HDL fractions. Within this time frame no significant differences were observed in plasma insulin (Fig. 3b) or glucose (Fig. 3c) concentrations between the group that was injected with apoAII as compared with the group that received apoAI. In the mice that were injected with apoAII, both the HDL and VLDL fractions had increased apoAII compared with mice injected with apoAI, with a greater increase in the HDL fraction (Fig. 3d). ApoAII injection also increased apoCII in VLDL, with a slight decrease in HDL. ApoAII injection had no effect on apoCIII of either VLDL or HDL compared with injection with apoAI. In the mice that were injected with apoAI, both VLDL and HDL showed an increase in apoAI, with the major increase in the HDL fraction (Fig. 3d).
FIGURE 3.
Acute effects of in vivo apoAII injection on plasma triglycerides, insulin, glucose, and apolipoprotein composition. Mouse apoAII and apoAI (used as a control) were isolated as described under “Experimental Procedures.” Following an overnight fast, 4 mg of either the apoAI or apoAII (columns indicated as +apoAI and +apoAII, respectively) were administered to C57BL/6J control mice via tail vein injection. Mice were bled from the retro-orbital plexus under isoflorane anesthesia just prior to injection (pre-injection) and at 4 h post-injection. Plasma triglyceride (a), insulin (b), and glucose (c) concentrations were determined as described under “Experimental Procedures.” Data represent the mean ± S.E. for 14 animals in each group for triglycerides and 6 animals in each group for insulin and glucose. *, values that are significantly different (p < 0.05) than control (+apoAI) mice. d, the plasma samples from six animals in each group, apoAI (AI) injected and apoAII (AII) injected, were pooled and the VLDL and HDL lipoprotein fractions isolated by ultracentrifugation as described under “Experimental Procedures.” Equal amounts of total VLDL and HDL protein were subjected to PAGE electrophoresis, and relative amounts of apoAII, apoAI, apoCII, and apoCIII were determined by Western blot analysis as described under “Experimental Procedures.” Differences in band intensity between the AI and AII groups for the same apolipoprotein reflect differences in protein mass. Differences in intensity between different apolipoproteins may not reflect actual differences in mass.
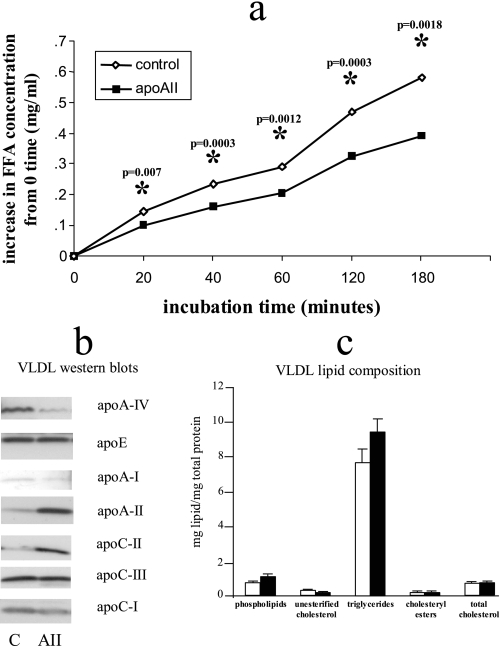
We previously demonstrated that the post-heparin plasma activities of both hepatic lipase and lipoprotein lipase were not significantly different in the apoAIItg mice (20, 21). To test whether the triglycerides in VLDL from the apoAIItg mice were less available for hydrolysis by lipoprotein lipase, we determined rates of triglyceride hydrolysis from ultracentrifugally isolated VLDL in vitro by purified lipoprotein lipase. Hydrolysis of VLDL from apoAIItg mice was significantly reduced compared with hydrolysis of VLDL from control animals (Fig. 4a). Western blot analysis demonstrated several differences between control and apoAIItg VLDL (Fig. 4b). ApoAII was present on VLDL from both groups, but the amount of apoAII was elevated ∼4 fold on VLDL from the apoAIItg mice (Fig. 4b). Significant differences in several other apolipoproteins were also confirmed by Western blot analysis. In addition to apoAII, VLDL from the apoAIItg mice clearly had increased amounts of apoCII, whereas the amount of apoAIV was significantly reduced. Small amounts of apoAI were also detected in the VLDLs with more in the control VLDL than apoAIItg VLDL. Control VLDL also had slightly increased apoCI compared with apoAIItg VLDL, whereas the amounts of apoCIII and apoE were similar between both groups (Fig. 4b).
FIGURE 4.
VLDL apolipoprotein and lipid composition and in vitro hydrolysis rates of VLDL triglycerides. VLDL (d < 1.0063) were isolated from plasma of overnight fasted control and apoAIItg mice by ultracentrifugation. a, VLDL at a final triglyceride concentration of 90 mg/dl were then incubated with isolated LPL (Sigma bovine milk LPL) at a final concentration of 24 μg/ml. Four replicates for each time point were incubated at 37 °C for 20, 40, 60, 120, and 180 min. At the end of each incubation period, the hydrolysis was stopped by adding 8 m urea to the reaction. Data represent the mean ± S.E. for the increase in free fatty acids above the 0 time values. *, values that are significantly different (p < 0.05) than control mice (S.E. bars are too small to be seen). b, isolated VLDLs were subjected to PAGE electrophoresis followed by Western blot analysis to determine the content of apolipoproteins, apoAII, apoAI, apoAIV, apoCI, apoCII, apoCIII, and apoE from control (C) and apoAIItg (AII) VLDL. Sample loading was normalized by total protein mass. Two sets of molecular weight markers were run on each gel as described under “Experimental Procedures” but are not included in the figure. These analyses were repeated three times on different batches of VLDL with very similar results each time. Data from a representative analysis are presented. Differences in intensity of each individual apolipoprotein reflect differences in protein content between C and AII groups. Differences in intensities between different apolipoproteins may not accurately reflect differences in their protein masses. c, analysis of phospholipids, unesterified cholesterol, triglycerides, cholesteryl esters, and total cholesterol was then determined on the isolated VLDLs as described under “Experimental Procedures.” Lipid data have been normalized by total VLDL protein, and values are presented as the mean ± S.E. from three separate VLDL preparations for each group.
In addition to changes in apolipoprotein content, we also determined the proportions of the various lipid classes in the ultracentrifugally isolated VLDLs. When normalized to total VLDL protein, the proportions of phospholipids, unesterified cholesterol, triglycerides, cholesteryl esters, and total cholesterol were similar between control and apoAIItg VLDL (Fig. 4c).
ApoAII Exchange between HDL and Triglyceride-rich Lipoproteins—During various stages of the metabolism of VLDL and HDL in the plasma compartment, several apolipoproteins are exchanged. During catabolism of the VLDL, surface components are transferred to subpopulations of HDL (62, 63). Also, VLDL is known to acquire certain apolipoproteins from HDL upon entering the plasma compartment, such as the apoCs, which regulate hydrolysis of lipoprotein triglycerides by LPL. The apoAII associated with HDL is reported to bind with higher affinity to the HDL particle compared with other HDL apolipoproteins such as apoAI and the apoCs (64, 65).
Exchange of apoAII between HDL and VLDL has received little attention. Because the majority of apoAII in plasma is associated with HDL, we tested whether incubation of VLDL with HDL, in vitro, could alter the apolipoprotein content of the VLDL and confer resistance to hydrolysis by lipoprotein lipase. We performed co-incubations of control VLDL and AII VLDL, with either PBS alone, PBS containing HDL from the same strain, or PBS containing HDL from the alternate strain. The VLDL were then re-isolated by gel filtration, and changes in apolipoprotein content and susceptibility to hydrolysis by LPL after the in vitro co-incubations were determined.
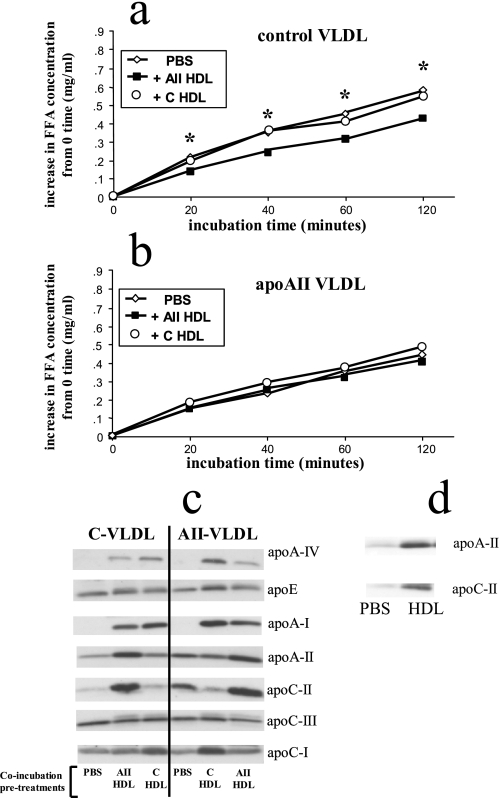
Control VLDL that had been incubated in vitro with apoAII HDL had significantly reduced rates of triglyceride hydrolysis compared with VLDL that had been incubated with buffer alone (Fig. 5a). In contrast, control VLDL that had been incubated in vitro with control HDL had rates of hydrolysis that were not significantly different than the VLDL incubated with buffer alone (Fig. 5a). Rates of hydrolysis of apoAII VLDL that were incubated with buffer alone were not significantly different after co-incubation with either apoAII HDL or control HDL (Fig. 5b).
FIGURE 5.
In vitro hydrolysis of VLDL triglycerides and changes in apolipoprotein composition after preincubation with HDL. VLDL (d < 1.0063) and HDL (d = 1.063-1.21) were isolated by density gradient ultracentrifugation from plasma of control and apoAIItg mice that had been fasted overnight. Aliquots of VLDL from both control mice (a) and apoAIItg mice (b) were incubated with PBS alone (PBS), HDL from the apoAIItg mice (+AII HDL), or HDL from control mice (+C HDL) at 37 °C for 1 h. The HDL and VLDL were then re-isolated by gel filtration chromatography using an Amersham Biosciences fast-protein liquid chromatography system. Aliquots of the VLDL re-isolated after the in vitro incubation with HDL were then tested in the in vitro hydrolysis assay as described in the legend of Fig. 4. Data represent the mean ± S.E. for the increase in free fatty acids above the 0 time values. There are four replicates at each time point for each group. *, values that are significantly different (p < 0.05) compared with VLDL incubated with PBS alone (S.E. bars are too small to be seen). c, apolipoproteins from the control VLDL (C-VLDL) and apoAIItg VLDL (AII-VLDL) from the various incubations were then separated by PAGE. Western blot analysis was performed as described under “Experimental Procedures” to determine changes in apolipoproteins apoAI, apoAII, apoAIV, apoCI, apoCII, apoCIII, and apoE after the incubations. Differences in band intensity among any of the lanes for each individual apolipoprotein represent actual differences in mass. Differences in band intensity between different apolipoproteins do not necessarily represent actual differences in mass. d, aliquots of the control VLDL inside 50-kDa molecular mass cutoff dialysis tubing were dialyzed against apoAIItg HDL (HDL) or PBS alone (PBS) for 12 h at room temperature as described under “Experimental Procedures.” The VLDL was then recovered and the mass of apoAII and CII determined by PAGE electrophoresis and Western blotting. A separate control was performed with only PBS in the dialysis tubing incubated against the apoAII HDL. No apoCII or apoAII could be detected in the PBS after 12-h incubation (data not shown).
We also determined the protein composition of VLDLs after incubation with the HDLs and observed several changes in the apolipoprotein profile (Fig. 5c). Both control VLDL and AII VLDL acquired more apoAII from AII HDL, but the increase was more pronounced for the control VLDL. In co-incubations with the control HDL, the apoAII content of control VLDL was slightly increased, whereas the apoAII content of AII VLDL decreased. Even though control HDL reduced the amount of apoAII associated with the apoAII VLDL, the apoAII content was still higher than in control VLDL that had been incubated with either PBS alone or with control HDL. Control VLDL and AII VLDL both acquired more apoAI from both AII HDL and control HDL; however, both VLDLs acquired more apoAI from control HDL than AII HDL (Fig. 5c).
Incubation with control HDL increased the amount of apoE on apoAIItg VLDL and slightly decreased the amount on control VLDL. Incubation with apoAIItg HDL had little effect on the amount of apoE associated with either control or apoAIItg VLDL (Fig. 5c).
Both control and apoAIItg VLDL acquired substantial apoCII from AII HDL. After incubation with control HDL apoCII content of control VLDL was relatively unchanged, whereas AII VLDL lost apoCII after incubation with control HDL (Fig. 5c). ApoCIII content of both control and AII VLDLs were relatively unchanged after incubation with any of the HDLs.
The amount of apoCI associated with both control and AII VLDLs were relatively unchanged after incubation with AII HDL, but both had more after incubation with control HDL. Both control VLDL and AII VLDL acquired apoAIV from both control HDL and AII HDL, but both VLDLs acquired more from the control HDL than the AII HDL (Fig. 5c).
In the experiments described above, the various apolipoproteins were differentially transferred between lipoprotein classes, suggesting that the complex transfer profile was not due to aggregation of HDL with VLDL during the co-incubations. Furthermore, gel filtration analysis of lipoprotein fractions is a well documented methodology that has been clearly demonstrated to separate VLDL from HDL. However, to be sure that apoAII does indeed transfer from HDL to VLDL, we performed another set of co-incubations of ultracentrifugally isolated control VLDL with ultracentrifugally isolated AII HDL. In these co-incubations the lipoprotein fractions were separated by a 50-kDa molecular mass cutoff dialysis tubing. Following incubation, the apoAII and apoCII content of the VLDL was significantly increased, even though the VLDL and HDL were separated by dialysis tubing (Fig. 5d). As an additional control to be certain that intact HDL was not passing through the membrane, PBS without VLDL was incubated inside the dialysis tubing, with the apoAII HDL on the outside. No apoAII or apoCII was detected in the PBS (data not shown).
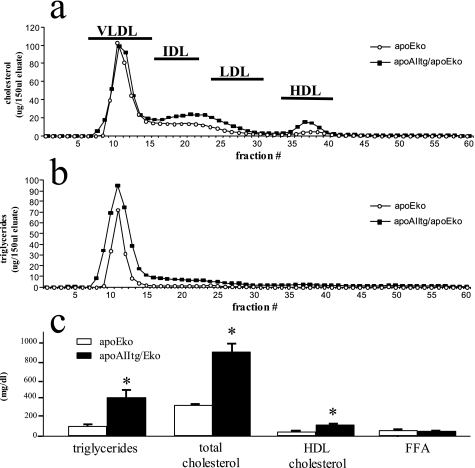
Small amounts of apoAII/apoE complexes had been reported on VLDL. To determine if the hypertriglyceridemia in the apoAIItg mice was somehow due to this putative interaction, the mouse apoAII transgene was bred onto an apoEko background. Although there were some differences in the phenotype associated with loss of apoE, the combined apoAIItg/apoEko mice exhibited a significant hypertriglyceridemia compared with the apoEko mice, with an increase in VLDL triglycerides (Fig. 6).
FIGURE 6.
Plasma lipid profile of apoEko and combined apoAIItg/apoEko mice. Plasma was pooled from 12 apoEko and apoAIItg/apoEko mice that had been maintained on a standard chow diet and fasted overnight. The lipoprotein fractions were separated by gel filtration using an Amersham Biosciences fast-protein liquid chromatography system as described under “Experimental Procedures.” Cholesterol (a) and triglyceride (b) concentrations were determined in each fraction. Plasma lipid concentrations (c) were also determined as described under “Experimental Procedures.” Data represent the mean ± S.E. for 12 animals in each group. *, values significantly different (p < 0.05) than apoEko mice.
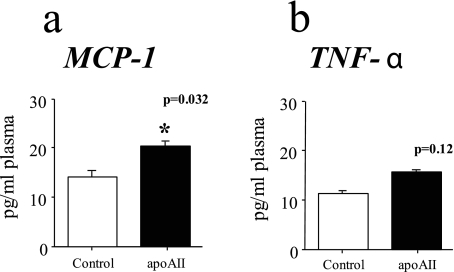
ApoAII Increases Oxidative Stress—Increased oxidative stress has been linked to insulin resistance in a variety of ways. We previously demonstrated that HDL from the apoAIItg mice are pro-inflammatory (3). As a measure of overall oxidative stress, we determined the plasma concentrations of TNF-α and MCP-1 (Fig. 7). MCP-1 concentrations were significantly increased in the plasma of the apoAIItg mice (Fig. 7a), and there was a trend toward increased TNF-α as well (Fig. 7b).
FIGURE 7.
Plasma concentrations of MCP-1 and TNF-α. MCP-1 (a) and TNF-α (b) plasma concentrations were determined by enzyme-linked immunosorbent assay on plasma from C57BL/6J control or apoAIItg mice that had been maintained on a standard chow diet and fasted overnight. Data represent the mean ± S.E. for 15 animals in each group. *, values that are significantly different (p < 0.05) than control mice.
DISCUSSION
Overproduction of VLDL is considered to be a primary mechanism linking increased plasma triglyceride concentrations with obesity and insulin resistance (28-33). Consistent with this hypothesis, our present studies provide a mechanism linking increased apoAII expression with increased VLDL secretion in the context of a mouse model which also exhibits increased adiposity and insulin resistance. We also demonstrated an acute inhibitory effect of apoAII on the hydrolysis of VLDL and chylomicron triglycerides, which appears to precede the development of insulin resistance. Furthermore, we demonstrated that apoAII can transfer from HDL to VLDL in vitro, making the VLDL a poorer substrate for hydrolysis by lipoprotein lipase. The fact that apoAII is not synthesized in intestine (66), yet increased hepatic expression of apoAII dramatically retarded chylomicron metabolism, argues strongly that apoAII is transferred to triglyceride rich lipoproteins in vivo. Taken together our results indicate that one function of apoAII is to modulate the metabolism of triglyceride-rich lipoproteins, with HDL serving as a pool of apoAII, as with the apoC apolipoproteins. The development of skeletal muscle insulin resistance in the apoAII transgenic mice may not be due to increased FFA obtained from the elevated plasma triglyceride concentrations. However, the elevated plasma VLDL concentrations, as well as other alterations in the plasma lipoproteins in the apoAIItg mice, may contribute to an increased inflammatory response promoting the development of skeletal muscle insulin resistance. These points are discussed below.
The apoAIItg mice exhibited increased rates of hepatic triglyceride secretion (Fig. 1, a and b). Furthermore, the increase in hepatic triglyceride secretion was due to increased rates of lipogenesis (Fig. 1d) and shunting of fatty acids into the pathways of esterification and away from oxidation (Fig. 1c). These are all metabolic changes that would be expected as a normal response of the liver to the chronically increased glucose and insulin concentrations in the apoAIItg mice (28-33) and are consistent with several aspects of the hepatic phenotype that we have already reported (20, 21). However, the magnitude of the increase in secretion did not appear sufficient to completely account for the marked hypertriglyceridemia in the apoAIItg mice.
We also demonstrated that rates of chylomicron clearance were markedly reduced in the apoAIItg mice (Fig. 2) and that VLDL from the apoAIItg mice was a poorer substrate for hydrolysis by purified lipoprotein lipase in vitro (Fig. 4a). Decreased clearance of triglyceride-rich lipoproteins is thus likely to be the major factor promoting hypertriglyceridemia in this animal model. This observation is consistent with the increased rates of remnant clearance that has been reported in apoAII knockout mice (21) as well as decreased rates of VLDL and chylomicron clearance observed in studies of mice expressing a transgene for human apoAII (13, 18, 67, 68).
The observation, that injection of purified mouse apoAII was able to acutely increase plasma triglyceride concentrations (Fig. 3a), suggests a direct role for apoAII in the regulation of triglyceride metabolism. This finding is supported by the recent observations that injection of human apoAII into rabbits or mice, either as artificial lipoproteins or in a lipid free state, also increased VLDL triglycerides (19, 69).
Several studies have demonstrated that purified human apoAII can associate with human and mouse VLDL and that such enriched VLDL are less efficiently hydrolyzed by lipoprotein lipase (19, 67, 70). In the present study we have in addition demonstrated that apoAII can transfer from HDL to VLDL in vitro (Fig. 5c) and that the resulting VLDL is a poorer substrate for hydrolysis by lipoprotein lipase (Fig. 5a). We do not know if more apoAII is secreted on nascent VLDL in the apoAII transgenic mice. However, because mouse intestine does not synthesize apoAII (66), the decreased clearance of chylomicrons in the apoAIItg mice in vivo (Fig. 2) was likely due to chylomicrons acquiring apoAII from HDL in the plasma. Thus, HDL may serve as a plasma reservoir of apoAII that is transferred to the triglyceride-rich lipoproteins in much the same way as VLDL and chylomicrons acquire most of their apoCs from HDL. Interestingly, an early study reported transfer of apoAI and apoAII from HDL to VLDL in vitro upon hydrolysis of the VLDL triglycerides (71). Taken together, these results suggest that metabolic perturbations that increase the amount of apoAII associated with VLDL might then be expected to increase plasma triglyceride concentrations. Such a mechanism may contribute, in part, to the complex disorder familial combined hyperlipidemia (48). Also, the VLDL of Tangiers patients is enriched with apoAII and is a relatively poor substrate for hydrolysis by lipoprotein lipase (72). A similar enrichment of VLDL with apoAII has also been observed in patients with type V hyperlipidemia (72). Most recently, an apoAII polymorphism was found to regulate postprandial clearance of a saturated fat overload in healthy men (73).
Little is known about the orientation of apoAII bound to VLDL that would suggest how it might be inhibiting hydrolysis by LPL. Although there are a few reports of apoAII forming complexes with apoE on VLDL (74, 75), this is unlikely to be the mechanism, because we have demonstrated that placing the transgene on an apoE knockout background still results in a significant increase in VLDL triglycerides (Fig. 6). Also, although apoE is important for receptor-mediated uptake of lipoprotein remnants from the plasma, there is no evidence to suggest that apoE interferes with the hydrolysis of triglycerides by LPL. The apoCs, on the other hand, are known to affect hydrolysis of plasma triglycerides. Most notably, apoCII is a cofactor that is required for full activity of lipoprotein lipase (76). Although some apoCII is secreted on VLDL, the majority of apoCII is acquired by VLDL in the plasma where it is transferred from HDL (77, 78). However, the apoCII content of VLDL from our apoAIItg mice was significantly increased compared with control VLDL (Fig. 4b). This is consistent with the findings of another study in which VLDL from mice expressing a transgene for human apoAII also had increased apoCII associated with it (67). In contrast to apoCII, apoCIII is known to inhibit hydrolysis of VLDL triglycerides, and apoCIII transgenic mice are hypertriglyceridemic (79). However, the amounts of apoCIII were similar between control and apoAIItg VLDLs. The decreased rates of hydrolysis of apoAII VLDL do not appear to be due to a decrease in apoCII or an increase in apoCIII. Other studies have demonstrated that apoAII interferes with the hydrolysis of HDL triglycerides by hepatic lipase (13, 81) and remodeling of HDL by endothelial lipase (82), neither of which require apoCII as a cofactor nor are inhibited by apoCIII. Therefore, apoAII appears able to impair the activity of several members of the lipase family, on different types of lipoproteins, through a mechanism independent of apoCs.
Our studies clearly show that the hypertriglyceridemia in mice overexpressing mouse apoAII is due to multiple factors acting in concert to both increase secretion of VLDL by the liver and decrease clearance of triglyceride rich lipoproteins. Although studies of transgenic mice expressing human apoAII, as compared with mouse apoAII, have resulted in variable findings with respect to various aspects of the complex insulin-resistant syndrome phenotype we observe (5, 6, 13, 20, 21, 67, 83), most have demonstrated hypertriglyceridemia (11-18, 67).
The effects in the apoAIItg mice appear to be physiologic. Mice with a null mutation for apoAII exhibit increased insulin sensitivity and increased rates of remnant lipoprotein clearance, the opposite of what we observed by overexpressing apoAII (22). Furthermore, we previously demonstrated, in a mouse cross with the apoAIItg mice, that plasma triglyceride, FFA, glucose, and insulin concentrations are significantly correlated with plasma apoAII concentrations over the entire physiologic and supra-physiologic range observed in the plasma (20).
In our initial characterization of the apoAIItg model we observed insulin resistance associated with increased fat mass, as well as increased triglycerides and free fatty acid levels (21). Our initial hypothesis was that the adipose tissue was insulin-resistant, releasing more free fatty acids into the plasma, which were then likely to induce insulin resistance in skeletal muscle as well as drive VLDL secretion by the liver. However, we demonstrated that release of FFA from adipose tissue was actually reduced by ∼50%, consistent with a normal response to the increased insulin and glucose, which is also likely to be the main factor promoting the increase in fat mass in the apoAIItg mice (20). We then focused on the skeletal muscle and confirmed that it was insulin-resistant, exhibiting not only defects in glucose metabolism but in fatty acid metabolism as well (20). Skeletal muscle fatty acid oxidation was reduced in the apoAIItg mice (20). Consistent with this decrease we also demonstrated that expression of medium-chain acyl-CoA dehydrogenase was significantly reduced. Interestingly, there was also a trend toward decreased expression of FABP and FATP-1, which are involved in the uptake and intracellular transport of FFA, respectively (20). This suggests that uptake of FFA may also be reduced in the skeletal muscle of the apoAIItg mice. Uptake of free fatty acids has been reported to be diminished in insulin-resistant skeletal muscle (84, 85). Because of the total mass of skeletal muscle, even modestly decreased rates of uptake could contribute to the elevated plasma FFA concentrations in the apoAII transgenic mice.
In the present study we have demonstrated increased VLDL secretion (Fig. 1), most likely as a normal response of the liver to the chronically elevated insulin and glucose, which derives from the skeletal muscle insulin resistance. This is also consistent with our observation that rosiglitazone treatment improved the insulin resistance and hypertriglyceridemia (20). In the present study we also demonstrated that, in addition to increased secretion from the liver, hydrolysis of VLDL and chylomicron triglycerides was impaired (Figs. 2 and 4). At steady-state concentrations clearance rates must match the increased secretion rates. Thus, even though the rates of hydrolysis are decreased, because of increased secretion by the liver, the overall fatty acid supply available to skeletal muscle may be increased. Increasing fatty acid to skeletal muscle has been linked to the development of skeletal muscle insulin resistance, as discussed above. However, if the increased triglyceride secretion, and thus the available FFA supply to skeletal muscle, is increased as a consequence of insulin resistance, then what factors initiate the development of the skeletal muscle insulin resistance?
In a recent study, VLDL was shown to activate components of the mitogen-activated protein kinase/NFκB signaling cascade (86), pre-conditioning the cells such that they were much more sensitive in their response to the pro-inflammatory cytokine, TNFα. This mechanism did not involve extracellular hydrolysis of the VLDL triglycerides but did require receptor-mediated uptake of the VLDL (86). Interestingly, this effect was specific for VLDL, because it was not observed with chylomicrons. In the present study we have demonstrated that apoAII acutely inhibits hydrolysis of VLDL, increasing plasma concentrations before insulin resistance develops. Thus, increased VLDL could contribute to increased cellular responses to oxidative stress.
Oxidative stress is recognized as an important factor contributing to insulin resistance (40, 87). One of the important links between obesity and skeletal muscle insulin resistance are proinflammatory cytokines released by adipose tissue, such as TNFα, which have been demonstrated to induce insulin resistance in skeletal muscle (80). In addition to a putative role for VLDL in the apoAIItg mice, we have previously demonstrated increased oxidative stress in this animal model, with HDL that are pro-inflammatory rather than anti-inflammatory, and which is likely to contribute to the increased atherosclerosis. When added directly to co-cultures of human aortic endothelial and smooth muscle cells, HDL from the apoAIItg mice induced inflammatory responses such as increased MCP-1 production (3). In the present study we have also demonstrated that plasma concentrations of MCP-1 are significantly elevated (Fig. 7a).
The function of apoAII has been a mystery. Our previous studies showed effects on HDL function and metabolism. Our present studies, and the work of others, now suggest that one function of apoAII is to directly modulate VLDL lipolysis. Our studies also suggest mechanisms that link hyperlipidemia, insulin resistance, and obesity in an animal model that is likely to have relevance to the insulin-resistant syndrome in humans. Several studies have demonstrated that increased apoAII has similar effects in humans (10, 36-39, 41-47). The complex nature of the phenotype underscores the need for relevant animal models to investigate and understand complex diseases such as the insulin resistance syndrome.
This work was supported by National Institutes of Health Grants HL28481 and DK071673. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: apoAII, apolipoprotein AII; HDL, high density lipoprotein; LDL, low density lipoprotein; VLDL, very low density lipoprotein; FFA, free fatty acid; PPARγ, peroxisome proliferator-activated receptor-γ; PBS, phosphate-buffered saline; LPL, lipoprotein lipase; TNF, tumor necrosis factor.
References
- 1.Schonfeld, G., Bailey, A., and Steelman, R. (1978) Lipids 12 951-959 [DOI] [PubMed] [Google Scholar]
- 2.Tall, A. R. (1998) Eur. Heart J. 19 Suppl. A, A31-A35 [PubMed] [Google Scholar]
- 3.Castellani, L. W., Navab, M., Van Lenten, B. J., Hedrick, C. C., Hama, S. Y., Goto, A. M., Fogelman, A. M., and Lusis, A. J. (1997) J. Clin. Invest. 100 464-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbaras, R., Puchois, P., Fruchart, J. C., and Ailhaud, G. (1987) Biochem. Biophys. Res. Commun. 142 63-69 [DOI] [PubMed] [Google Scholar]
- 5.Warden, C. H., Hedrick, C. C., Qiao, J. H., Castellani, L. W., and Lusis, A. J. (1993) Science 261 469-472 [DOI] [PubMed] [Google Scholar]
- 6.Hedrick, C. C., Castellani, L. W., Warden, C. H., Puppione, D. L., and Lusis, A. J. (1993) J. Biol. Chem. 268 20676-20682 [PubMed] [Google Scholar]
- 7.Schultz, J. R., Verstuyft, J. G., Gong, E. L., Nichols, A. V., and Rubin, E. M. (1993) Nature 365 762-764 [DOI] [PubMed] [Google Scholar]
- 8.Mehrabian, M., Qiao, J. H., Hyman, R., Ruddle, D., Laughton, C., and Lusis, A. J. (1993) Arterioscler. Thromb. 13 1-10 [DOI] [PubMed] [Google Scholar]
- 9.Warden, C. H., Daluiski, A., Bu, X., Purcell-Huynh, D. A., De Meester, C., Shieh, B. H., Puppione, D. L., Gray, R. M., Reaven, G. M., and Chen, Y. D. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 10886-10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vohl, M. C., Lamarche, B., Bergeron, J., Moorjani, S., Prud'homme, D., Nadeau, A., Tremblay, A., Lupien, P. J., Bouchard, C., and Despres, J. P. (1997) Atherosclerosis 128 183-190 [DOI] [PubMed] [Google Scholar]
- 11.Kalopissis, A. D., Pastier, D., and Chambaz, J. (2003) Curr. Opin. Lipidol. 14 165-172 [DOI] [PubMed] [Google Scholar]
- 12.Tailleux, A., Duriez, P., Fruchart, J. C., and Clavey, V. (2002) Atherosclerosis 164 1-13 [DOI] [PubMed] [Google Scholar]
- 13.Zhong, S., Goldberg, I. J., Bruce, C., Rubin, E., Breslow, J. L., and Tall, A. (1994) J. Clin. Invest. 94 2457-2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzal-Casacuberta, A., Blanco-Vaca, F., Ishida, B. Y., Julve-Gil, J., Shen, J., Calvet-Marquez, S., Gonzalez-Sastre, F., and Chan, L. (1996) J. Biol. Chem. 271 6720-6728 [DOI] [PubMed] [Google Scholar]
- 15.Julve-Gil, J., Ruiz-Perez, E., Casaroli-Marano, R. P., Marzal-Casacuberta, A., Escola-Gil, J. C., Gonzalez-Sastre, F., and Blanco-Vaca, F. (1999) Metabolism 48 415-421 [DOI] [PubMed] [Google Scholar]
- 16.Escola-Gil, J. C., Marzal-Casacuberta, A., Julve-Gil, J., Ishida, B. Y., Ordonez-Llanos, J., Chan, L., Gonzalez-Sastre, F., and Blanco-Vaca, F. (1998) J. Lipid Res. 39 457-462 [PubMed] [Google Scholar]
- 17.Julve, J., Escola-Gil, J. C., Marzal-Casacuberta, A., Ordonez-Llanos, J., Gonzalez-Sastre, F., and Blanco-Vaca, F. (2000) Biochim. Biophys. Acta 1488 233-244 [DOI] [PubMed] [Google Scholar]
- 18.Escola-Gil, J. C., Julve, J., Marzal-Casacuberta, A., Ordonez-Llanos, J., Gonzalez-Sastre, F., and Blanco-Vaca, F. (2001) J. Lipid Res. 42 241-248 [PubMed] [Google Scholar]
- 19.Dugue-Pujol, S., Rousset, X., Pastier, D., Quang, N. T., Pautre, V., Chambaz, J., Chabert, M., and Kalopissis, A. D. (2006) J. Lipid Res. 47 2631-2639 [DOI] [PubMed] [Google Scholar]
- 20.Castellani, L. W., Gargalovic, P., Febbraio, M., Charugundla, S., Jien, M. L., and Lusis, A. J. (2004) J. Lipid Res. 45 2377-2387 [DOI] [PubMed] [Google Scholar]
- 21.Castellani, L. W., Goto, A. M., and Lusis, A. J. (2001) Diabetes 50 643-651 [DOI] [PubMed] [Google Scholar]
- 22.Weng, W., and Breslow, J. L. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 14788-14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharrett, A. R., Ballantyne, C. M., Coady, S. A., Heiss, G., Sorlie, P. D., Catellier, D., and Patsch, W. (2001) Circulation 104 1108-1113 [DOI] [PubMed] [Google Scholar]
- 24.Drexel, H., Amann, F. W., Beran, J., Rentsch, K., Candinas, R., Muntwyler, J., Luethy, A., Gasser, T., and Follath, F. (1994) Circulation 90 2230-2235 [DOI] [PubMed] [Google Scholar]
- 25.Packard, C. J., Demant, T., Stewart, J. P., Bedford, D., Caslake, M. J., Schwertfeger, G., Bedynek, A., Shepherd, J., and Seidel, D. (2000) J. Lipid Res. 41 305-318 [PubMed] [Google Scholar]
- 26.Mahley, R. W., and Huang, Y. J. (2007) Clin. Invest. 117 94-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira, L. D., Pulawa, L. K., Jensen, D. R., and Eckel, R. H. (2001) Diabetes 50 1064-1068 [DOI] [PubMed] [Google Scholar]
- 28.Ginsberg, H. N., Zhang, Y. L., and Hernandez-Ono, A. (2005) Arch. Med. Res. 36 232-240 [DOI] [PubMed] [Google Scholar]
- 29.Zammit, V. A. (2002) Ann. N. Y. Acad. Sci. 967 52-65 [DOI] [PubMed] [Google Scholar]
- 30.McGarry, J. D. (2002) Diabetes 51 7-18 [DOI] [PubMed] [Google Scholar]
- 31.Shillabeer, G., Hornford, J., Forden, J. M., Wong, N. C., Russell, J. C., and Lau, D. C. (1992) J. Lipid Res. 33 31-39 [PubMed] [Google Scholar]
- 32.Bazin, R., and Lavau, M. J. (1982) Lipid Res. 23 839-849 [PubMed] [Google Scholar]
- 33.Assimacopoulos-Jeannet, F., Brichard, S., Rencurel, F., Cusin, I., and Jeanrenaud, B. (1995) Metabolism 44 228-233 [DOI] [PubMed] [Google Scholar]
- 34.Brunmair, B., Gras, F., Neschen, S., Roden, M., Wagner, L., Waldhausl, W., and Furnsinn, C. (2001) Diabetes 50 2309-2315 [DOI] [PubMed] [Google Scholar]
- 35.Kim, S. Y., Kim, H. I., Park, S. K., Im, S. S., Li, T., Cheon, H. G., and Ahn, Y. H. (2004) Diabetes 53 Suppl. 1, S66-S70 [DOI] [PubMed] [Google Scholar]
- 36.van 't Hooft, F. M., Ruotolo, G., Boquist, S., de Faire, U., Eggertsen, G., and Hamsten, A. (2001) Circulation 104 1223-1228 [DOI] [PubMed] [Google Scholar]
- 37.Baier, L. J., Wiedrich, C., Dobberfuhl, A., Traurig, M., Thuillez, P., Bogardus, C., and Hanson, R. (1998) Diabetes 47 (suppl.) A171 [Google Scholar]
- 38.Elbein, S. C., Yount, P. A., Teng, K., and Hasstedt, S. J. (1998) Diabetes 47 A15 [Google Scholar]
- 39.Cerne, D., Kaplan-Pavlovcic, S., Kranjec, I., and Jurgens, G. (2000) Ren. Fail. 22 799-808 [DOI] [PubMed] [Google Scholar]
- 40.Schernthaner, G. H., and Schernthaner, G. (2005) Scand. J. Clin. Lab. Invest. Suppl. 240 30-40 [DOI] [PubMed] [Google Scholar]
- 41.Klos, K. L., Kardia, S. L., Ferrell, R. E., Turner, S. T., Boerwinkle, E., and Sing, C. F. (2001) Arterioscler. Thromb. Vasc. Biol. 21 971-978 [DOI] [PubMed] [Google Scholar]
- 42.Cerne, D., Ledinski, G., Kager, G., Greilberger, J., Wang, X., and Jurgens, G. (2000) Clin. Chem. Lab. Med. 38 529-538 [DOI] [PubMed] [Google Scholar]
- 43.Lara-Castro, C., Hunter, G. R., Lovejoy, J. C., Gower, B. A., and Ferna′ndez, J. R. (2005) Obesity Res. 13 507-512 [DOI] [PubMed] [Google Scholar]
- 44.Fager, G., Wiklund, O., Olofsson, S. O., Wilhelmsson, C., and Bondjers, G. (1980) Atherosclerosis 36 67-74 [DOI] [PubMed] [Google Scholar]
- 45.Pilger, E., Prsitantz, A., Pfeiffer, K. P., and Kostner, G. (1983) Arteriosclerosis 3 57-63 [DOI] [PubMed] [Google Scholar]
- 46.Aleksandrovich, O. V., Ozerova, I. N., Olfer'ev, A. M., Serdyuk, A. P., Metel'skaya, V. A., and Perova, N. V. (2006) Bull. Exp. Biol. Med. 141 678-681 [DOI] [PubMed] [Google Scholar]
- 47.Corella, D., Arnett, D. K., Tsai, M. Y., Kabagambe, E. K., Peacock, J. M., Hixson, J. E., Straka, R., Province, M., Lai, C. Q., Parnell, L. D., Borecki, I., and Ordovas, J. M. (2007) Clin. Chem. 53 1144-1152 [DOI] [PubMed] [Google Scholar]
- 48.Allayee, H., Castellani, L. W., Cantor, R. M., de Bruin, T. W., and Lusis, A. J. (2003) Circ. Res. 92 1262-1267 [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro, A., Pastier, D., Kardassis, D., Chambaz, J., and Cardot, P. (1999) J. Biol. Chem. 274 1216-1225 [DOI] [PubMed] [Google Scholar]
- 50.Pajukanta, P., Lilja, H. E., Sinsheimer, J. S., Cantor, R. M., Lusis, A. J., Gentile, M., Duan, X. J., Soro-Paavonen, A., Naukkarinen, J., Saarela, J., Laakso, M., Ehnholm, C., Taskinen, M. R., and Peltonen, L. (2004) Nat. Genet. 36 371-376 [DOI] [PubMed] [Google Scholar]
- 51.Puppione, D. L., and Charugundla, S. (1994) Lipids 29 595-597 [DOI] [PubMed] [Google Scholar]
- 52.Soler-Argilaga, C., Russell, R. L., and Heimberg, M. (1977) Arch. Biochem. Biophys. 178 135-139 [DOI] [PubMed] [Google Scholar]
- 53.Olubadewo, J., Morgan, D. W., and Heimberg, M. (1983) J. Biol. Chem. 258 938-945 [PubMed] [Google Scholar]
- 54.Folch, J., Lees, M., and Sloane-Stanley, G. H. (1957) J. Biol. Chem. 226 497-509 [PubMed] [Google Scholar]
- 55.Castellani, L. W., Wilcox, H. C., and Heimberg, M. (1991) Biochim. Biophys. Acta 1086 197-208 [DOI] [PubMed] [Google Scholar]
- 56.Lowenstein, J. M., Brunengraber, H., and Wadke, M. (1975) Methods Enzymol. 35 279-287 [DOI] [PubMed] [Google Scholar]
- 57.LeBoeuf, R. C., Puppione, D. L., Schumaker, V. N., and Lusis, A. J. (1983) J. Biol. Chem. 258 5063-5070 [PubMed] [Google Scholar]
- 58.Mills, J. L., Tuomilehto, J., Yu, K. F., Colman, N., Blaner, W. S., Koskela, P., Rundle, W. E., Forman, M., Toivanen, L., and Rhoads, G. G. (1992) J. Pediatr. 120 863-871 [DOI] [PubMed] [Google Scholar]
- 59.Redlich, C. A., Graue, J. N., Van Bennekum, A. M., Clever, S. L., Ponn, R. B., and Blaner, W. S. (1996) Am. J. Respir. Crit. Care Med. 154 1436-1443 [DOI] [PubMed] [Google Scholar]
- 60.Blaner, W. S., Obunike, J. C., Kurlandsky, S. B., al-Haideri, M., Piantedosi, R., Deckelbaum, R. J., and Goldberg, I. (1994) J. Biol. Chem. 269 16559-16565 [PubMed] [Google Scholar]
- 61.Burger, H., Kovacs, A., Weiser, B., Grimson, R., Nachman, S., Tropper, P., van Bennekum, A. M., Elie, M. C., and Blaner, W. S. (1997) J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 14 321-326 [DOI] [PubMed] [Google Scholar]
- 62.Magill, P., Rao, S. N., Miller, N. E., Nicoll, A., Brunzell, J., St Hilaire, J., and Lewis, B. (1982) Eur. J. Clin. Invest. 12 113-120 [DOI] [PubMed] [Google Scholar]
- 63.Eisenberg, S., Patsch, J. R., Sparrow, J. T., Gotto, A. M., and Olivecrona, T. (1979) J. Biol. Chem. 254 12603-12608 [PubMed] [Google Scholar]
- 64.Rosseneu, M., Van Tornout, P., Caster, H., and Lievens, M. J. (1981) Acta Cardiol. Suppl. 27 11-29 [PubMed] [Google Scholar]
- 65.Rye, K. A., Wee, K., Curtiss, L. K., Bonnet, D. J., and Barter, P. J. (2003) J. Biol. Chem. 278 22530-225366 [DOI] [PubMed] [Google Scholar]
- 66.LeBoeuf, R. C., Doolittle, M. H., Montcalm, A., Martin, D. C., Reue, K., and Lusis, A. J. (1990) J. Lipid Res. 31 91-101 [PubMed] [Google Scholar]
- 67.Boisfer, E., Lambert, G., Atger, V., Tran, N. Q., Pastier, D., Benetollo, C., Trottier, J. F., Beaucamps, I., Antonucci, M., Laplaud, M., Griglio, S., Chambaz, J., and Kalopissis, A. D. (1999) J. Biol. Chem. 274 11564-11572 [DOI] [PubMed] [Google Scholar]
- 68.Escola-Gil, J. C., Julve, J., Marzal-Casacuberta, A., Ordonez-Llanos, J., Gonzalez-Sastre, F., and Blanco-Vaca, F. (2000) J. Lipid Res. 41 1328-1338 [PubMed] [Google Scholar]
- 69.Hime, N. J., Drew, K. J., Wee, K., Barter, P. J., and Rye, K. A. (2006) J. Lipid Res. 47 115-122 [DOI] [PubMed] [Google Scholar]
- 70.Mao, S. J., Kluge, K., and Squillace, S. J. (1981) FEBS Lett. 132 289-292 [DOI] [PubMed] [Google Scholar]
- 71.Musliner, T. A., Michenfelder, H. J., and Krauss, R. M. (1988) J. Lipid Res. 29 349-361 [PubMed] [Google Scholar]
- 72.Alaupovic, P., Knight-Gibson, C., Wang, C. S., Downs, D., Koren, E., Brewer, H. B., Jr., and Gregg, R. E. (1991) J. Lipid Res. 32 9-19 [PubMed] [Google Scholar]
- 73.Delgado-Lista, J., Perez-Jimenez, F., Tanaka, T., Perez-Martinez, P., Jimenez-Gomez, Y., Marin, C., Ruano, J., Parnell, L., Ordovas, J. M., and Lopez-Miranda, J. (2007) J. Nutr. 137 2024-2028 [DOI] [PubMed] [Google Scholar]
- 74.Borghini, I., James, R. W., Blatter, M. C., and Pometta, D. (1991) Biochim. Biophys. Acta 1083 139-146 [DOI] [PubMed] [Google Scholar]
- 75.Tozuka, M., Hidaka, H., Miyachi, M., Furihata, K., Katsuyama, T., and Kanai, M. (1992) Biochim. Biophys. Acta 1165 61-67 [DOI] [PubMed] [Google Scholar]
- 76.Ekman, R., and Nilsson-Ehle, P. (1975) Clin. Chim. Acta 63 29-35 [DOI] [PubMed] [Google Scholar]
- 77.Goldberg, I. J., Scheraldi, C. A., Yacoub, L. K., Saxena, U., and Bisgaier, C. L. (1990) J. Biol. Chem. 265 4266-4272 [PubMed] [Google Scholar]
- 78.Havel, R. J., Kane, J. P., and Kashyap, M. L. (1973) J. Clin. Invest. 52 32-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ito, Y., Azrolan, N., O'Connell, A., Walsh, A., and Breslow, J. L. (1990) Science 249 790-793 [DOI] [PubMed] [Google Scholar]
- 80.Hotamisligil, G. S., Shargill, N. S., and Spiegelman, B. M. (1993) Science 259 87-91 [DOI] [PubMed] [Google Scholar]
- 81.Hedrick, C. C., Castellani, L. W., Wong, H., and Lusis, A. J. (2001) J. Lipid. Res. 2 563-570 [PubMed] [Google Scholar]
- 82.Caiazza, D., Jahangiri, A., Rader, D. J., Marchadier, D., and Rye, K. A. (2004) Biochemistry 43 11898-11905 [DOI] [PubMed] [Google Scholar]
- 83.Escola-Gil, J. C., Blanco-Vaca, F., and Julve, J. (2002) Diabetologia 45 600-601 [DOI] [PubMed] [Google Scholar]
- 84.Colberg, S. R., Simoneau, J. A., Thaete, F. L., and Kelley, D. E. (1995) J. Clin. Invest. 95 1846-1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turpeinen, A. K., Takala, T. O., Nuutila, P., Axelin, T., Luotolahti, M., Haaparanta, M., Bergman, J., Hamalainen, H., Iida, H., Maki, M., Uusitupa, M. I., and Knuuti, J. (1999) Diabetes 48 1245-1250 [DOI] [PubMed] [Google Scholar]
- 86.Ting, H. J., Stice, J. P., Schaff, U. Y., Hui, D. Y., Rutledge, J. C., Knowlton, A. A., Passerini, A. G., and Simon, S. I. (2007) Circ. Res. 100 381-390 [DOI] [PubMed] [Google Scholar]
- 87.Savage, D. B., Petersen, K. F., and Shulman, G. I. (2005) Hypertension 45 828-833 [DOI] [PubMed] [Google Scholar]