Abstract
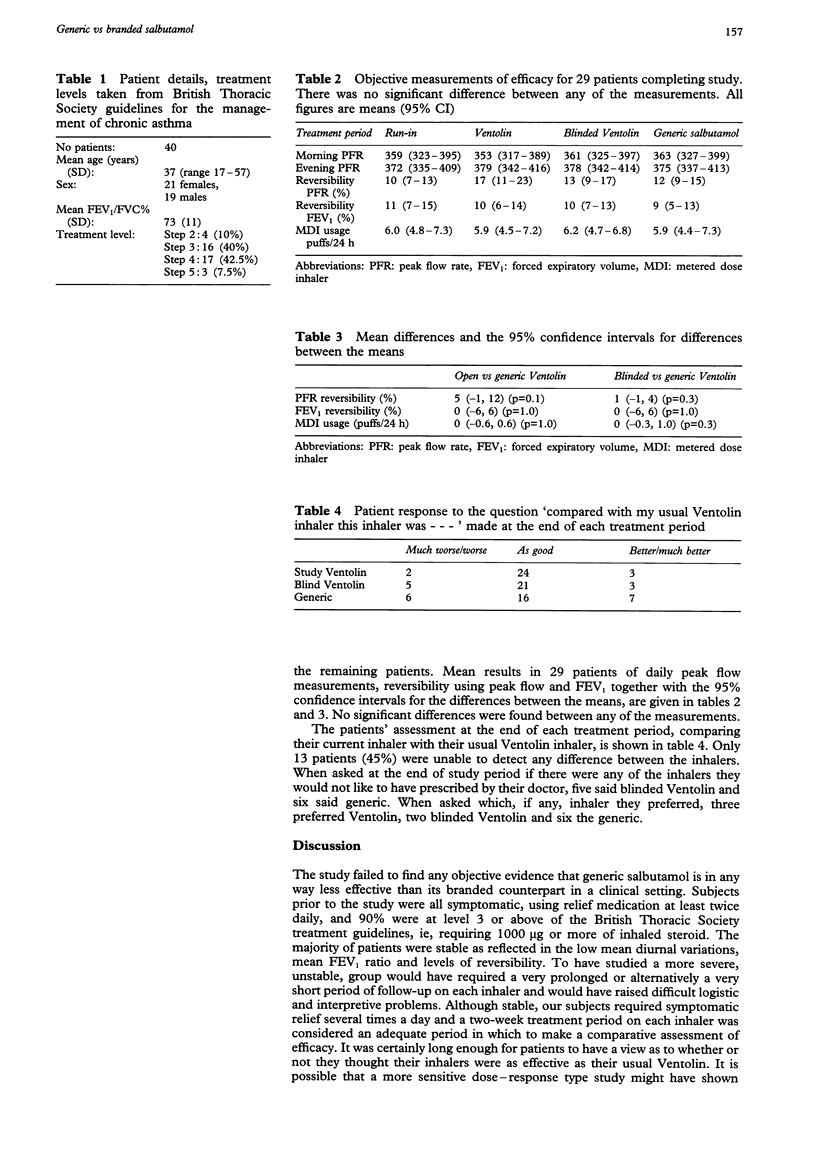
Generic substitution of salbutamol lags behind that of other drugs in Scotland and a negative perception by both patients and doctors may explain this. The aim of this study was to assess whether, in clinical practice, there was any difference in efficacy between branded salbutamol (Ventolin) and a generic preparation. Asthmatic patients using a Ventolin metered-dose inhaler at least twice a day for symptom relief were entered into a double-blind cross-over study, comparing Ventolin, blinded Ventolin and a generic salbutamol in random order for two weeks each. Daily peak flows, inhaler use and bronchodilator response were recorded. At the end of each treatment period patients rated their inhaler against their usual Ventolin on a 5-point scale. Forty patients were entered into the study; 90% received 1000 micrograms or more of inhaled steroids per day. Eleven patients dropped out during the run-in phase. In the remaining 29 patients, no significant difference between treatments could be found in any of the objective parameters measured. Fifty-five per cent of patients said they could detect a difference between the inhalers, and 45% noted a difference between their usual Ventolin and the open or blinded Ventolin. This study showed clinical equivalence between a generic and branded salbutamol. Patients' own assessment of their relief inhaler seems to be influenced by factors other than efficacy. The study highlights that careful encouragement is required when changing to a generic product and has particular implications for the forthcoming conversion to CFC-free products.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Pearson M., Lewis R., Watson J., Ayres J., Ibbotson G., Ryan D., Flynn D., Williams J. Generic inhalers for asthma. BMJ. 1994 Nov 26;309(6966):1440–1440. doi: 10.1136/bmj.309.6966.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart S. C., Custovic A., Richards D. H., Woodcock A. GR106642X: a new, non-ozone depleting propellant for inhalers. BMJ. 1995 Jun 24;310(6995):1639–1640. doi: 10.1136/bmj.310.6995.1639a. [DOI] [PMC free article] [PubMed] [Google Scholar]


