Abstract
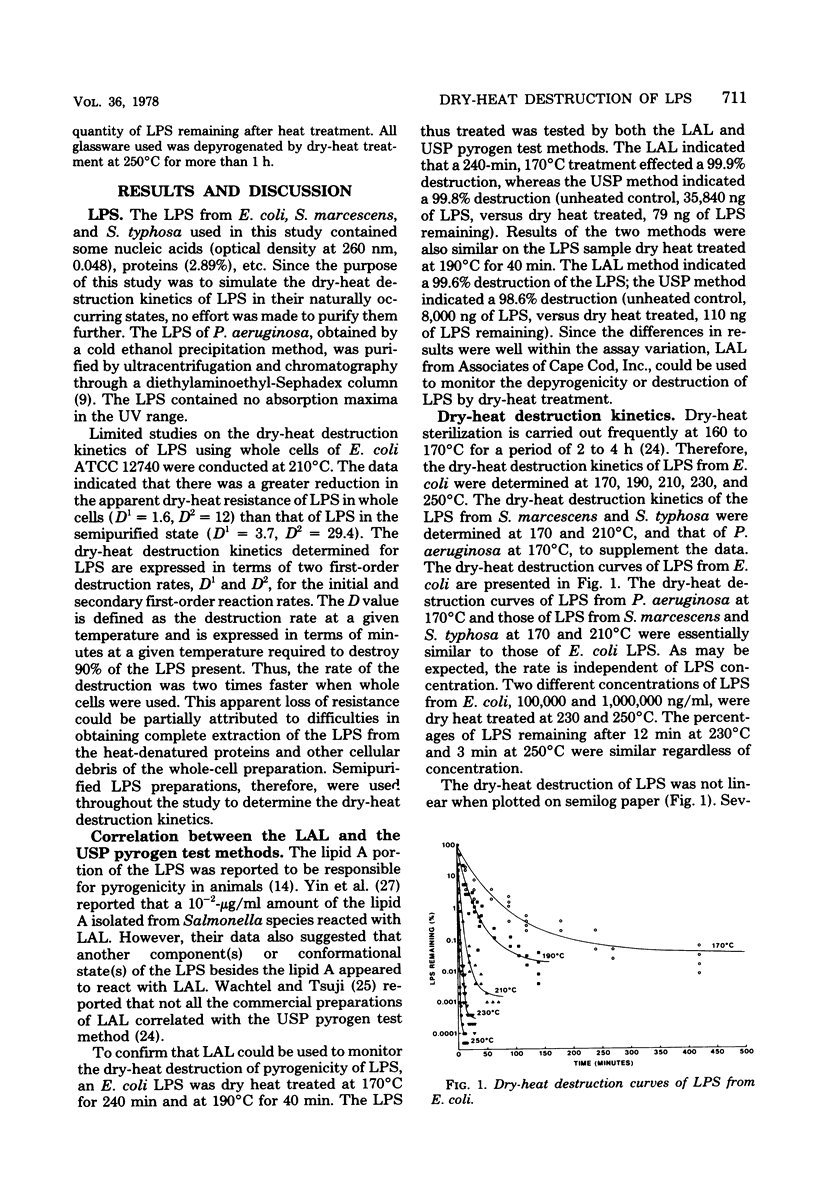
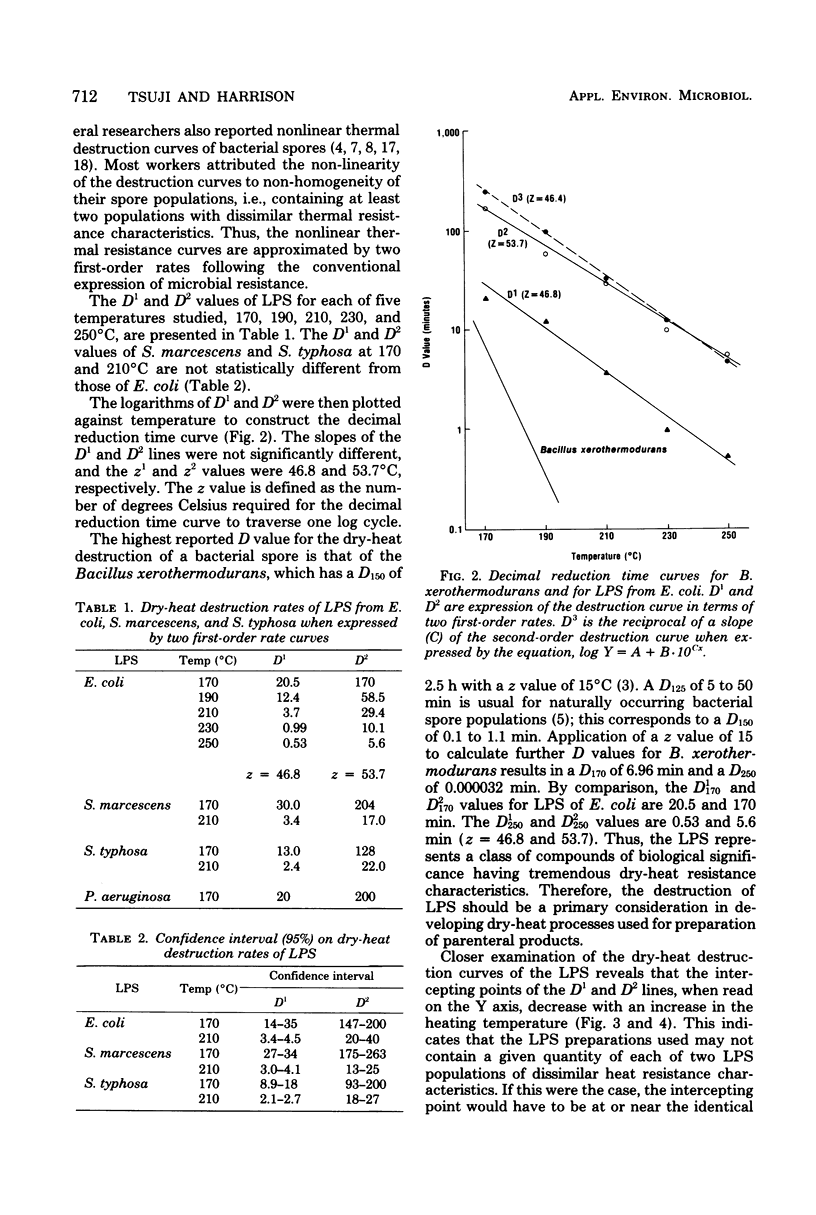
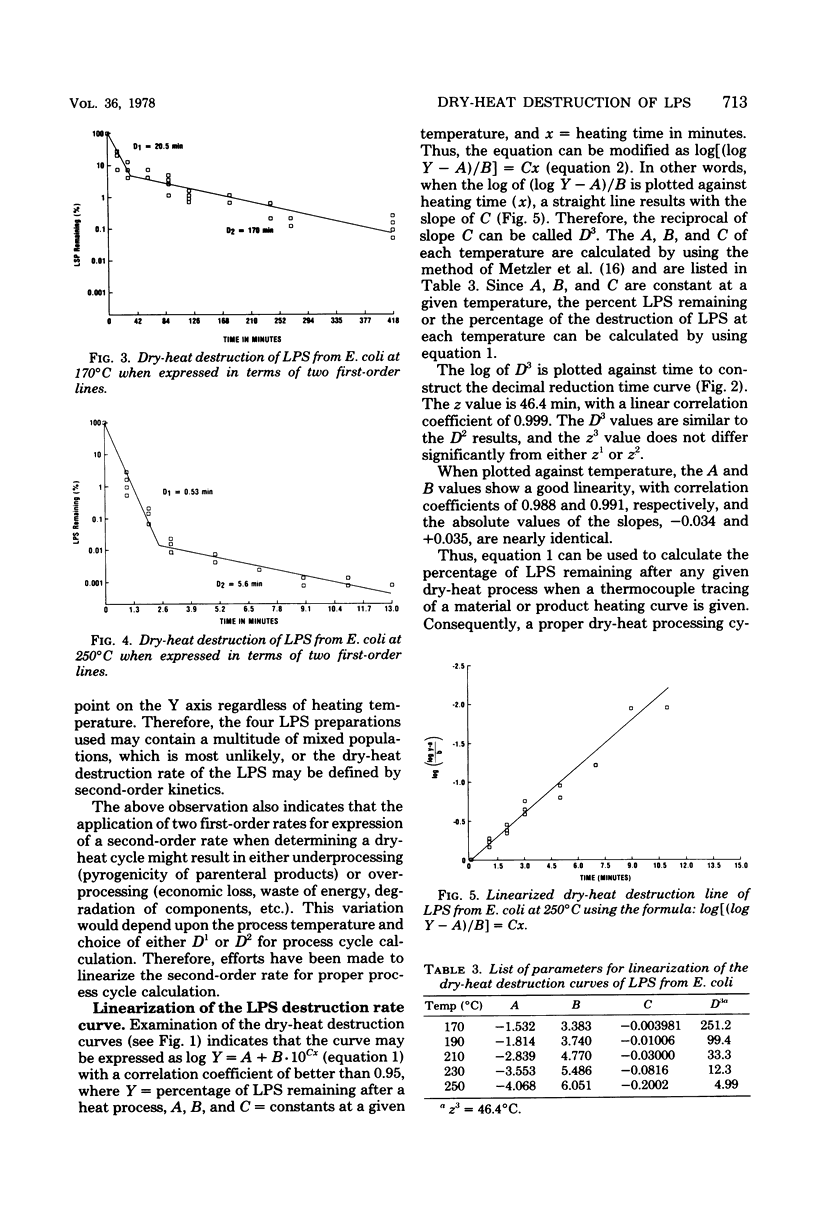
Dry-heat destruction kinetics of lipopolysaccharides from Escherichia coli, Serratia marcescens, and Salmonella typhosa at 170 to 250 degrees C are described. The destruction rate seems to follow the second order and can be linearized by the equation, log y = a + b . -10cx. Because c is the slope, 1/c = D3. Both a and b are constant at a given temperature and are linear functions of temperature. The D(3)170, D(3)190, D(3)210, D(3)230, and D(3)250 values for E. coli lipopolysaccharide are 251, 99.4, 33.3, 12.3, and 4.99 min, respectively, with a z value of 46.4 min. The D values for lipopolysaccharides from S. marcescens and S. typhosa are not significantly different from those from E. coli lipopolysaccharide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANG F. B. A bacterial disease of Limulus polyphemus. Bull Johns Hopkins Hosp. 1956 May;98(5):325–351. [PubMed] [Google Scholar]
- Barnett J. A., Sanford J. P. Bacterial shock. JAMA. 1969 Sep 8;209(10):1514–1517. [PubMed] [Google Scholar]
- Bond W. W., Favero M. S., Petersen N. J., Marshall J. H. Dry-heat inactivation kinetics of naturally occurring spore populations. Appl Microbiol. 1970 Oct;20(4):573–578. doi: 10.1128/am.20.4.573-578.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond W. W., Favero M. S., Petersen N. J., Marshall J. H. Relative frequency distribution of d(125 C) values for spore isolates from the mariner-Mars 1969 spacecraft. Appl Microbiol. 1971 May;21(5):832–836. doi: 10.1128/am.21.5.832-836.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond W. W., Favero M. S. Thermal profile of a Bacillus species (ATCC 27380) extremely resistant to dry heat. Appl Microbiol. 1975 Jun;29(6):859–860. doi: 10.1128/am.29.6.859-860.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK H. A., CAMPBELL L. L., Jr The nonlogarithmic rate of thermal destruction of spores of Bacillus coagulans. Appl Microbiol. 1957 Jul;5(4):243–248. doi: 10.1128/am.5.4.243-248.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K., Pflug I. J. Effect of temperature and gas velocity on dry-heat destruction rate of bacterial spores. Appl Microbiol. 1968 Feb;16(2):343–348. doi: 10.1128/am.16.2.343-348.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanessian S., Regan W., Watson D., Haskell T. H. Isolation and characterization of antigenic components of a new heptavalent Pseudomonas vaccine. Nat New Biol. 1971 Feb 17;229(7):209–210. doi: 10.1038/newbio229209a0. [DOI] [PubMed] [Google Scholar]
- Hase S., Rietschel E. T. Isolation and analysis of the lipid A backbone. Lipid A structure of lipopolysaccharides from various bacterial groups. Eur J Biochem. 1976 Mar 16;63(1):101–107. doi: 10.1111/j.1432-1033.1976.tb10212.x. [DOI] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- McKAY D. G., SHAPIRO S. S., SHANBERGE J. N. Alterations in the blood coagulation system induced by bacterial endotoxins. II. In vitro. J Exp Med. 1958 Mar 1;107(3):369–376. doi: 10.1084/jem.107.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin G., Ostilund K. Dry-heat inactivation of Bacillus subtilis spores by means of infra-red heating. Antonie Van Leeuwenhoek. 1975;41(3):329–335. doi: 10.1007/BF02565067. [DOI] [PubMed] [Google Scholar]
- Reinhold R. B., Caridis D. T., Fine J. Diagnosis of clinical endotoxemia. J Reprod Med. 1972 Jun;8(6):335–339. [PubMed] [Google Scholar]
- Rietschel E. T. Chemical structure and biological activity of endotoxins (lipopolysaccharides) and lipid A. Naunyn Schmiedebergs Arch Pharmacol. 1975;287(1):73–84. doi: 10.1007/BF00632639. [DOI] [PubMed] [Google Scholar]
- Robertson J. H., Gleason D., Tsuji K. Dry-heat destruction of lipopolysaccharide: design and construction of dry-heat destruction apparatus. Appl Environ Microbiol. 1978 Nov;36(5):705–709. doi: 10.1128/aem.36.5.705-709.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon; certain factors involved in the production of the local hemorrhagic necrosis. J Exp Med. 1951 May;93(5):489–504. doi: 10.1084/jem.93.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel R. E., Tsuji K. Comparison of limulus amebocyte lysates and correlation with the United States Pharmacopeial pyrogen test. Appl Environ Microbiol. 1977 Jun;33(6):1265–1269. doi: 10.1128/aem.33.6.1265-1269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weary M., Baker B. Utilization of the limulus amebocyte lysate test for pyrogen testing large volume parenterals, administration sets, and medical devices. Bull Parenter Drug Assoc. 1977 May-Jun;31(3):127–133. [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]


