Abstract
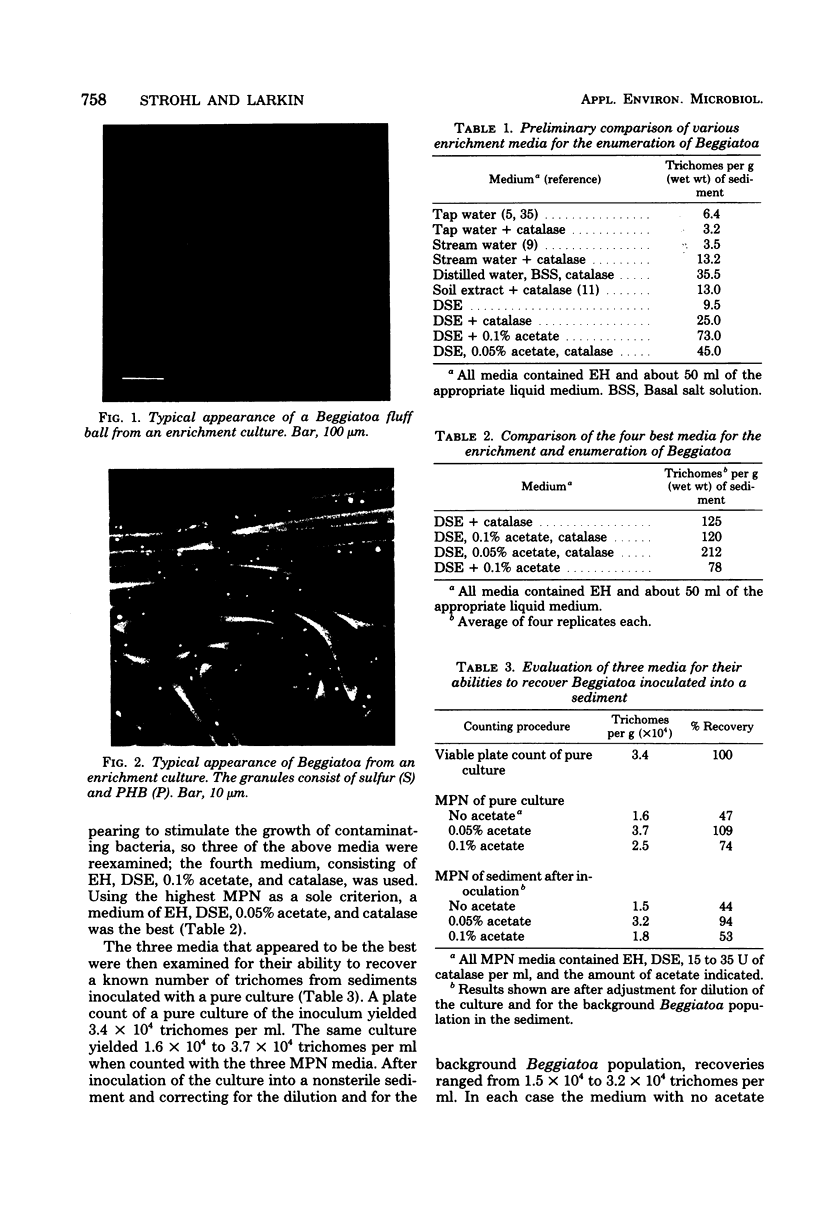
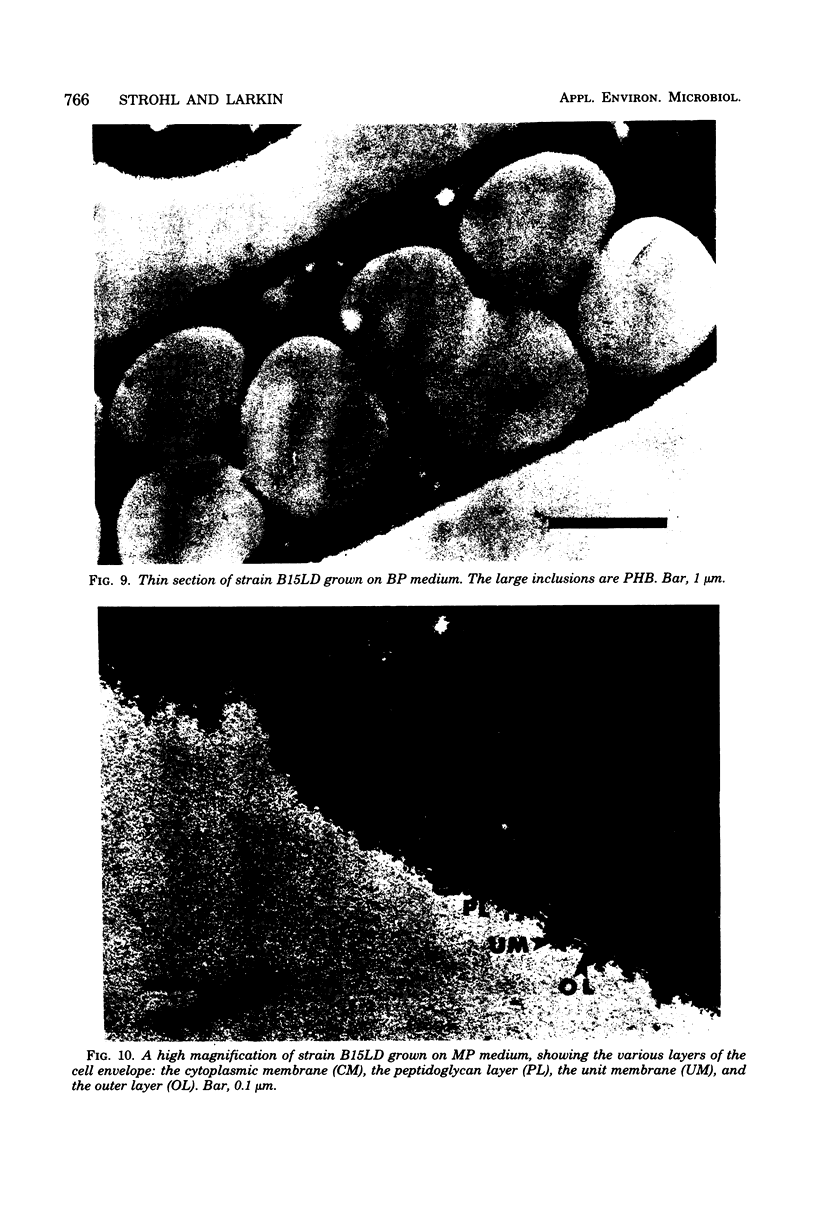
An accurate most-probable-number enumeration method was developed for counting the number of Beggiatoa trichomes from various freshwater sediments. The medium consisted of extracted hay, diluted soil extract, 0.05% acetate, and 15 to 35 U of catalase per ml. The same enrichment medium, but without the acetate, was the best enrichment medium from which to obtain pure cultures because it supported good growth of the beggiatoas without allowing them to be overgrown by other bacteria. A total of 32 strains of Beggiatoa were isolated from seven different freshwater habitats and partially characterized. The strains were separated into five groups based on several preliminary characteristics. Four of the groups contained cells with trichomes of approximately the same diameter (1.5 to 2.7 μm) and may be Beggiatoa leptomitiformis or an unnamed species. The fifth group appeared to be Beggiatoa alba. With the exception of three strains, all of the strains deposited sulfur in the presence of hydrogen sulfide, and all strains grew heterotrophically and deposited poly-β-hydroxybutyrate and volutin when grown on acetate supplemented with low concentrations of other organic nutrients. Thin sections of sulfur-bearing trichomes indicated that the sulfur granules were external to the cytoplasmic membrane and that they were surrounded by an additional membrane.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON S. D., MORITA R. Y. EFFECT OF CATALASE AND CULTURAL CONDITIONS ON GROWTH OF BEGGIATOA. J Bacteriol. 1964 Dec;88:1755–1761. doi: 10.1128/jb.88.6.1755-1761.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton S. D., Morita R. Y., Miller W. Utilization of acetate by Beggiatoa. J Bacteriol. 1966 Mar;91(3):1192–1200. doi: 10.1128/jb.91.3.1192-1200.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W. E., Jr, Biggins J. Respiratory mechanisms in the Flexibacteriaceae: terminal oxidase systems of Saprospira grandis and Vitreoscilla species. J Bacteriol. 1971 Mar;105(3):1083–1089. doi: 10.1128/jb.105.3.1083-1089.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch R. N., Hageage G. J. Motility in procaryotic organisms: problems, points of view, and perspectives. Biol Rev Camb Philos Soc. 1968 Aug;43(3):317–362. doi: 10.1111/j.1469-185x.1968.tb00963.x. [DOI] [PubMed] [Google Scholar]
- FAUST L., WOLFE R. S. Enrichment and cultivation of Beggiatoa alba. J Bacteriol. 1961 Jan;81:99–106. doi: 10.1128/jb.81.1.99-106.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar G. J., Boyle W. C. Occurrence of filamentous microorganisms in activated sludge. J Water Pollut Control Fed. 1971 May;43(5):779–798. [PubMed] [Google Scholar]
- Garlick S., Oren A., Padan E. Occurrence of facultative anoxygenic photosynthesis among filamentous and unicellular cyanobacteria. J Bacteriol. 1977 Feb;129(2):623–629. doi: 10.1128/jb.129.2.623-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M. M., Hollis J. P. Interaction of beggiatoa and rice plant: detoxification of hydrogen sulfide in the rice rhizosphere. Science. 1977 Jan 14;195(4274):179–180. doi: 10.1126/science.195.4274.179. [DOI] [PubMed] [Google Scholar]
- Joshi M. M., Hollis J. P. Rapid enrichment of Beggiatoa from soil. J Appl Bacteriol. 1976 Apr;40(2):223–224. doi: 10.1111/j.1365-2672.1976.tb04169.x. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S., Murray R. G. The fine structure of Thioploca ingrica and a comparison with Beggiatoa. Can J Microbiol. 1965 Aug;11(4):645–655. doi: 10.1139/m65-087. [DOI] [PubMed] [Google Scholar]
- Muller L. L., Jacks T. J. Rapid chemical dehydration of samples for electron microscopic examinations. J Histochem Cytochem. 1975 Feb;23(2):107–110. doi: 10.1177/23.2.1117127. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Schmidt G. L. Structure of the Chromatium sulfur particle and its protein membrane. J Bacteriol. 1971 Mar;105(3):1142–1148. doi: 10.1128/jb.105.3.1142-1148.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts G., Allam A. I., Hollis J. P. Beggiatoa: occurrence in the rice rhizosphere. Science. 1972 Dec 1;178(4064):990–992. doi: 10.1126/science.178.4064.990. [DOI] [PubMed] [Google Scholar]
- Pringsheim E. G. Die Mixotrophie von Beggiatoa. Arch Mikrobiol. 1967;59(1):247–254. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- SCOTEN H. L., STOKES J. L. Isolation and properties of Beggiatoa. Arch Mikrobiol. 1962;42:353–368. doi: 10.1007/BF00409071. [DOI] [PubMed] [Google Scholar]
- SKERMAN V. B., DEMENTJEVA G., CAREY B. J. Intracellular deposition of sulfur by Sphaerotilus natans. J Bacteriol. 1957 Apr;73(4):504–512. doi: 10.1128/jb.73.4.504-512.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH-WHITE S., PEACOCK W. J., TURNER B., DEN DULK G. M. A ring chromosome in man. Nature. 1963 Jan 5;197:102–103. doi: 10.1038/197102b0. [DOI] [PubMed] [Google Scholar]
- Schmidt G. L., Nicolson G. L., Kamemd Composition of the sulfur particle of Chromatium vinosum strain D. J Bacteriol. 1971 Mar;105(3):1137–1141. doi: 10.1128/jb.105.3.1137-1141.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Skerman V. B. Bacterial diversity: the natural history of selected morphologically unusual bacteria. Annu Rev Microbiol. 1965;19:407–454. doi: 10.1146/annurev.mi.19.100165.002203. [DOI] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. Respiratory chain of colorless algae. II. Cyanophyta. Plant Physiol. 1966 Apr;41(4):599–605. doi: 10.1104/pp.41.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]