Abstract
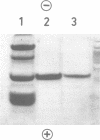
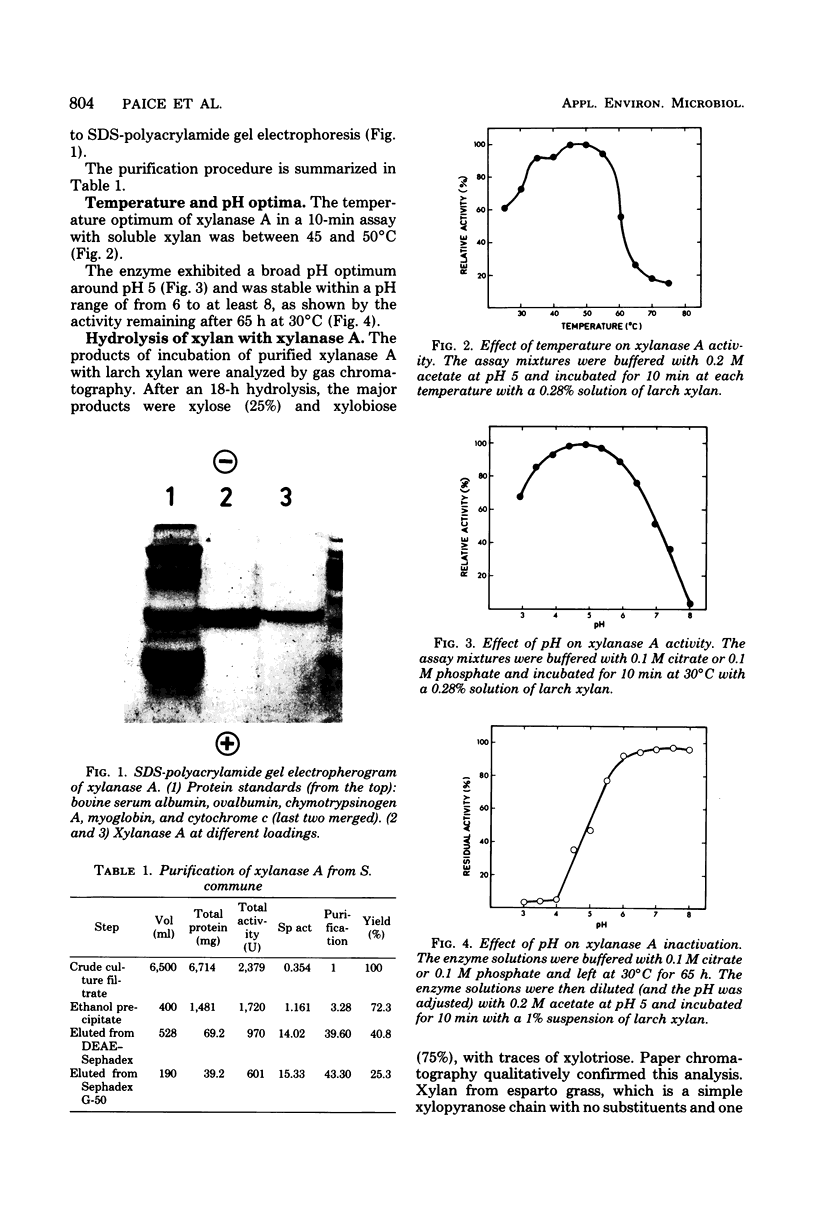
Xylanase A, one of several extracellular xylanases produced by Schizophyllum commune strain Delmar when grown in submerged culture with spruce sawdust as carbon source, was purified 43-fold in 25% yield with respect to total xylanase activity. Although some polysaccharide was strongly bound to the purified enzyme, the complex could be dissociated by sodium dodecyl sulfate and appeared homogeneous on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The molecular weight of the protein, calculated from the electrophoretic mobility, was 33,000. The molecular activity of the purified xylanase A, determined with soluble larch xylan as substrate, was 1.4 X 10(5) min-1, with xylobiose and xylose as the major products. The enzyme had a pH optimum of 5.0 and a temperature optimum of 55 degrees C in 10-min assays. The acid hydrolysate of xylanase A was rich in aspartic acid and aromatic amino acids. The sequence of 27 residues at the amino terminus showed no homology with known sequences of other proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish-Bowden A. Assessment of protein sequence identity from amino acid composition data. J Theor Biol. 1977 Apr 21;65(4):735–742. doi: 10.1016/0022-5193(77)90019-4. [DOI] [PubMed] [Google Scholar]
- Dekker R. F., Richards G. N. Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem. 1976;32:277–352. doi: 10.1016/s0065-2318(08)60339-x. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Edman P. Sequence determination. Mol Biol Biochem Biophys. 1970;8:211–255. doi: 10.1007/978-3-662-12834-3_8. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 1. Separation, purification and physico-chemical characterization of five endo-1,4-beta-glucanases. Eur J Biochem. 1975 Feb 3;51(1):193–206. doi: 10.1111/j.1432-1033.1975.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Gorbacheva I. V., Rodionova N. A. Studies on xylan degrading enzymes. I. Purification and characterization of endo-1,4-beta-xylanase from Aspergillus niger str. 14. Biochim Biophys Acta. 1977 Sep 15;484(1):79–93. doi: 10.1016/0005-2744(77)90114-0. [DOI] [PubMed] [Google Scholar]
- Gum E. K., Jr, Brown R. D., Jr Comparison of four purified extracellular 1,4-beta-D-glucan cellobiohydrolase enzymes from Trichoderma viride. Biochim Biophys Acta. 1977 May 27;492(1):225–231. doi: 10.1016/0005-2795(77)90229-x. [DOI] [PubMed] [Google Scholar]
- Hurst P. L., Sullivan P. A., Shepherd M. G. Chemical modification of cellulase from Aspergillus niger. Biochem J. 1977 Dec 1;167(3):549–556. doi: 10.1042/bj1670549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurásek L., Colvin J. R., Whitaker D. R. Microbiological aspects of the formation and degradation of cellulosic fibers. Adv Appl Microbiol. 1967;9:131–170. doi: 10.1016/s0065-2164(08)70527-6. [DOI] [PubMed] [Google Scholar]
- Khalifah R. G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971 Apr 25;246(8):2561–2573. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Nakayama M., Tomita Y., Suzuki H., Nisizawa K. Partial proteolysis of some cellulase components from Trichoderma viride and the substrate specificity of the modified products. J Biochem. 1976 May;79(5):955–966. doi: 10.1093/oxfordjournals.jbchem.a131163. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Richards G. N., Shambe T. Production and purification of two hemicellulases from Cephalosporium sacchari. Carbohydr Res. 1976 Jul;49:371–381. doi: 10.1016/s0008-6215(00)83154-7. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Thoma J. A. A possible mechanism for amylase catalysis. J Theor Biol. 1968 Jun;19(3):297–310. doi: 10.1016/0022-5193(68)90141-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]