Abstract
Cadaveric renal transplants suffer frequently from delayed graft function, which is associated with increased risk for long-term graft survival loss. One-third of kidney grafts that are stored in current organ preservation solutions experience delayed graft function, demonstrating the urgent need for improvement. Although ischaemic graft injury is complex in nature, complement activation is considered important to the process. Here we show that pharmacological targeting of the complement 5a receptor (C5aR) during cold ischaemia has a protective effect on early kidney graft survival, inflammation and apoptosis in a mouse model of syngeneic kidney transplantation. Graft survival of kidneys that were stored in University of Wisconsin solution in the presence of a C5aR antagonist increased from 29% to 57%. Increased graft survival was associated with less tubular damage and apoptosis, protection from sustained C5aR expression and decreased production of tumour necrosis factor-α and macrophage inflammatory protein-2. In a translational approach, we determined C5aR expression in paediatric living-related and cadaveric allografts. C5aR expression was significantly higher in all compartments of kidneys from cadaveric compared with kidneys from living-related donors. C5aR expression in cadaveric kidneys correlated positively with cold ischaemia time, renal dysfunction and the frequency of apoptotic tubular cells, suggesting a novel role for C5a in delayed graft function pathogenesis. Supplementing organ preservation solutions with C5aR inhibitors may improve early graft function following cadaveric kidney transplantation.
Keywords: C5a, complement, experimental kidney transplantation, ischaemia/reperfusion injury, organ preservation
Introduction
Transplantation-associated ischaemia reperfusion injury (IRI) of cadaveric renal allografts drives delayed graft function (DGF) [1,2], a form of acute renal failure (ARF), resulting frequently in acute graft loss and/or chronic rejection [3]. Although the underlying pathomechanisms of renal IRI are complex, tubular epithelial cell damage at the corticomedullary junction is the main outcome of the injury [4], often associated with tubular cell apoptosis and/or necrosis [5].
The activation of the complement system by renal IRI and its role as a mediator of transplant injury is well appreciated (reviewed in [6,7]). Alternative pathway activation has been considered as the predominant pathway following renal IRI [8,9]. However, recent data point towards a major contribution of the lectin pathway [10,11], suggesting that the alternative pathway is critical to amplify lectin pathway-initiated complement activation [7]. Local production and activation of complement factors is of critical importance in triggering the generation of the complement 5a (C5a) anaphylatoxin [12,13] and the membrane attack complex, both of which can promote IRI-initiated tissue damage [14]. Accordingly, strategies aimed at prevention of complement activation or the specific blockade of complement pathways have the potential to protect from IRI-mediated tissue injury [15,16].
Organ preservation solutions are used currently to minimize renal tissue injury in cadaveric donor (CAD)-derived grafts during cold ischaemia (CI). In the United States, University of Wisconsin (UW) solution is the most frequently used preservation reagent [17]. Conceptually, such solutions are designed to reduce hypoxic and inflammatory injury as a more general approach to confine tissue damage. Because of the shortage in donor organs, the frequency of using grafts from marginal donors is rising, limiting the protective effect of organ preservation even more [18]. Further, non-heart-beating donors, who suffer from a high incidence of DGF in response to warm ischaemia, are used more frequently [19]. Importantly, the incidence of DGF is much lower in grafts from living-related donors (LRD) compared with CAD; however, the epidemic dimensions of renal failure in an ageing population of patients suggest that LRD will not solve the problem of organ shortage [20].
We hypothesized that combining the general protective effect of organ preservation with pharmacological targeting of the C5a/C5a receptor (C5aR) within the graft will result in improved graft protection during CI. To address this hypothesis experimentally we used the C5aR antagonist A8Δ71−773 (C5aRA), which targets both receptors for C5a, i.e. C5aR (CD88) and C5L2 [21]. Here, we demonstrate a positive regulatory impact of C5aRA on transplantation-associated IRI as evidenced by reduced cortical and medullar tissue damage, tissue inflammation and tubular apoptosis, resulting in increased graft survival. Further, we found strong positive correlations between C5aR expression in allografts from paediatric CAD and organ function, cold ischaemia time (CIT) and tubular apoptosis, suggesting a critical role for C5aR signalling in the development of DGF following paediatric kidney transplantation.
Materials and methods
Animal studies
All experiments involving animals were approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Hospital Research Foundation.
Kidney transplantation
Syngeneic kidney transplantation in C57BL/6 mice was performed as described previously [22]. Briefly, animals were anaesthetized with isoflurane and the left donor kidney attached to a cuff of the aorta and the renal vein with a small caval cuff, and the ureter were removed en bloc. Warm ischaemia time was 25–30 min. The donor kidney was perfused with UW preservation solution (groups A and B) or UW solution supplemented with C5aRA (group C) until the venous effluent was clear (∼1 ml). Subsequently, kidneys were placed in UW preservation solution for 30 min (group A) or 2 h in the absence (group B) or presence (group C) of C5aRA (10−6 M). After left nephrectomy of the recipient, the vascular cuffs were anastomosed to the recipient abdominal aorta and vena cava, respectively, below the level of the native renal vessels. The ureter was anastomosed directly into the bladder. Mice were killed 72 h after surgery by CO2 asphyxiation and both kidneys were removed for analysis.
Determination of tumour necrosis factor-α, macrophage inflammatory protein-2 and C5aR gene expression levels in kidney tissue
Quantification of gene expression was performed essentially as described [23]. Briefly, frozen kidney tissue was homogenized in Trizol reagent and RNA was extracted and quantified by spectrophotometry. After DNAse digestion, cDNA was obtained using superscript (RNaseH-) RTase and the mixtures were incubated for 50 min at 42°C followed by 15 min at 70°C and aliquots were then frozen. Standards for real-time reverse transcription–polymerase chain reaction (RT–PCR) were obtained from a macrophage cell line (J774.A1) stimulated for 2 h with lipopolysaccharide (200 ng/ml) at 37°C. Gene expression levels were determined by real-time RT–PCR using iQ-SYBRgreen reaction mix (Bio-Rad, Hercules, CA, USA) containing 5 μl cDNA and 500 nM primer. The following primers were used: glyceraldehye-3-phosphate-dehydrogenase forward: 5′-TGC ACC ACC AAC TGC TTA-3′, reverse: 5′-GGA TGC AGG GAT GAT GTT C-3′; tumour necrosis factor (TNF)-α forward: 5′-TTG TGG CAG GGG CCA CCA C-3′, reverse: 5′-GCC ATT TGG GAA CTT CTC ATC-3′; macrophage inflammatory protein (MIP)-2/CXCL2 forward: 5′-TCA GTG CTG CAC TGG TCC TG-3′, reverse: 5′-CAT TGA CAG CGC AGT TCA CTG-3′; C5aR forward: 5′-CAG GCG GTG TAG AGG AGA AG-3′, reverse: 5′-GAA GGA AGG AAG GAG GAG AGG-3′.
Apoptosis assay of mouse kidney tissue
To detect apoptotic nuclei, we used the In Situ Cell Death Detection Kit, POD (Roche Diagnostics GmbH, Penzberg, Germany) based on transferase-mediated dUTP nick-end labelling (TUNEL) according to the manufacturer's instructions. TUNEL-positive apoptotic nuclei were detected by fluorescence microscopy. Cells that displayed the characteristic morphology of apoptosis, including nuclear fragmentation, nuclear condensation and intensely fluorescent nuclei by TUNEL assay were considered apoptotic. In contrast, TUNEL-positive cells lacking morphological criteria were not considered apoptotic. Slides were examined in a blinded manner, and apoptosis was quantified by counting the number of TUNEL-positive nuclei per 100 tubular cells counted in an average of five high-power fields (×40) in each section.
Complement 5a receptor immunohistochemistry of mouse tissue
Cryostat sections (5 μm) were air-dried for 2 h and then treated with 3% H2O2/phosphate-buffered saline (PBS) : methanol (1 : 4) at room temperature for 20 min. After blocking, endogenous avidin and biotin (DakoCytomation Biotin Blocking System; Dako, Carpinteria, CA, USA) sections were covered with blocking buffer containing normal rabbit serum (5%), sodium azide (0·1%), human heat-aggregated immunoglobulin G (IgG) (1 mg/ml) in PBS for 30 min. Subsequently, sections were incubated with a rat monoclonal antibody (mAb) to mouse C5aR (5 μg/ml; clone 10/92; Hycult Biotechnology, Uden, the Netherlands) overnight at 4°C and then with a biotinylated anti-rat IgG (Vector Laboratories, Burlingame, CA, USA) for 90 min at room temperature. Slides were washed and then incubated with streptavidin–horesradish peroxidase (HRP) (1 : 2000 in PBS) for 45 min at room temperature. Substrate chromogen (Dako North America, Carpinteria, CA, USA), was added according to the manufacturer's directions and allowed to react for 2 min. Sections were counterstained with Meyer's haematoxylin for 1 min and then ‘blued’ in ammonia water for 30 s.
Haematoxylin and eosin staining and histological scoring
Serial sections (4 μm thickness) of paraffin embedded kidney tissue were dewaxed and rehydrated for staining with haematoxylin and eosin. The grade of histological damage was assigned by determining in a blinded fashion the extent of: haemorrhage, infiltrating cells and oedema − each on a grade of 0–3 (absent, mild, moderate and severe respectively). Spatial distribution of damage was determined by assessing the percentage area of tissue involved. The individual ‘tissue damage score’ and ‘tissue damage (%)’ values were then multiplied together to yield a measure of ‘relative kidney damage’.
Human biopsy material
We utilized excess protocol kidney biopsy samples obtained previously from allografts at 1 h of reperfusion after release of the vascular clamps during LRD (n = 13) or CAD (n = 12) kidney transplantation [24]. Paraffin-embedded kidney biopsy samples from transplant recipients who had consented previously to a study approved by the Institutional Review Board for examination of pathogenetic pathways were available for examination. Four-micron sections were cut onto slides and processed for routine haematoxylin and eosin histology, C5aR immunohistochemistry and TUNEL staining.
Complement 5a receptor immunohistochemistry on human biopsy material
Paraffin-embedded sections were deparaffinized and rehydrated through two changes of xylene and graded alcohols, fixed with fresh 4% formaldehyde in PBS for 30 min at 4°C, blocked with normal goat serum and incubated with anti-human C5aR mAb (clone W17/1; Hycult Biotechnology) for 1 h at room temperature. Slides were then exposed for 60 min to biotinylated anti-mouse secondary antibody, incubated for 30 min in HRP–streptavidin complex, developed for 5 min with HRP substrate mixture (ImmunoCruz Staining Systems, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and counterstained with haematoxylin. As a negative control, the primary antibody was omitted. C5aR staining was quantified in a blinded fashion by counting the number of positively stained cells in at least five high-power fields.
Apoptosis assay on human biopsy material
To quantify apoptosis, we performed the terminal deoxynucleotidedyl transferase (TUNEL) assay in paraffin wax-embedded sections as described previously [24] using the ApoAlert Assay Kit (Clontech, La Jolla, CA, USA), according to the manufacturer's instructions. Slides were examined in a blinded fashion and apoptosis was quantified as described above for mouse tissue.
Statistical evaluation
Statistical analysis was performed using the SigmaStat version 3·5 (SystatSoftware SSI, San Jose, CA, USA). All data are given as mean ± standard error of the mean. First we tested for a normal distributed population, using the Kolmogoroff–Smirnov test. Comparison of the means of more than two groups was performed by one-way analysis of variance. Pairwise comparison was performed using the Holm–Sidak method. Differences between treatment groups were considered significant with P < 0·05. Survival analysis was performed with Kaplan–Meier survival analysis using the log-rank test. Correlations between C5aR tissue staining in human biopsy samples and serum creatinine (sCr), CIT and frequency of tubular epithelial apoptosis were determined using Pearson's product–moment correlation.
Results
Pharmacological C5aR targeting improves early graft survival
To define the impact of C5aR blockade during CI, we established three different treatment groups. In group A, donor grafts were kept in UW preservation solution for 30 min. In groups B and C, CIT was extended to 2 h. In group C, C5aRA was added to the preservation solution. The different treatments had a substantial impact on graft survival. The loss of kidney grafts was defined as death of the animal. Animals that died within the first 6–8 h because of bleeding issues, i.e. a leaking anastomosis, were excluded from the study. During the first 24 h following transplantation, graft survival rates within the different treatment groups were between 68% (group A) and 71% (group C). Twenty-four to 48 h after transplantation, graft survival rates decreased substantially in groups A (from 68% to 36%) and B (from 67% to 24%). In contrast, we found only a modest decrease in graft survival in group C mice (from 71% to 57%) which did not change during the next 24 h (Fig. 1).
Fig. 1.
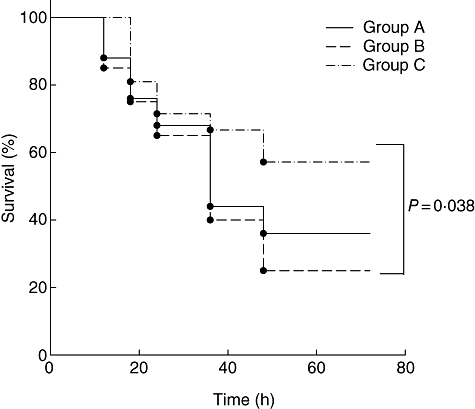
Impact of pharmacological complement 5a receptor (C5aR) targeting on kidney graft survival. Percentage survival rate of mice in each group as a function of post-transplantation time. Group A: 30 min cold ischaemia (CI) in University of Wisconsin (UW) solution (n = 25); group B: 2 h in UW solution without C5aRA (n = 21); group C: 2 h in UW solution with the addition of C5aRA (10−6 M) (n = 21). Pharmacological C5aR targeting improves graft survival rates significantly after 2 h of CI (P = 0·038; log-rank test).
Complement 5a receptor blockade reduces kidney damage
Kidney damage was evaluated in transplanted and non-transplanted kidneys 72 h after syngeneic kidney transplantation. CI for 30 min (group A) resulted in some glomerular changes, i.e. a slightly decreased Bowman's space, but relatively normal tubules were apparent (Fig. 2a; left panel). The contralateral kidney appeared normal (Fig. 2a; right panel). Dramatic changes were apparent in the transplanted kidney after 2 h CI (Fig. 2b; left panel). Glomeruli were avascular and acellular and tubules showed massive cellular disintegration. Large foci of haemorrhage and numerous oedematous regions were apparent throughout the tissue. Some damage was also apparent in the contralateral kidney (Fig. 2b; right panel), although it was less pronounced. Glomeruli were evident, but were dysmorphic and tubules were separated widely owing to significant oedema. Tissue damage in the transplanted kidney of group C mice was reduced significantly (Fig. 2c; left panel), as evidenced by the presence of glomeruli and reduced areas of haemorrhage. Generally, the tissue was similar in appearance to that in group A mice. In agreement with these findings, the contralateral kidney (Fig. 2c; right panel) appeared relatively normal in terms of glomerular and tubular structure, with no oedema.
Fig. 2.
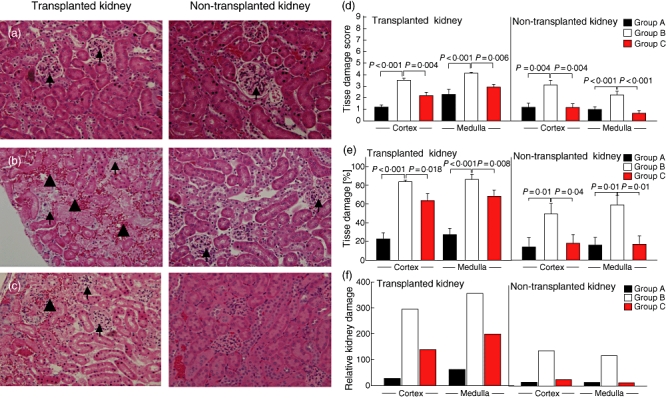
Impact of complement 5a receptor (C5aR) targeting on ischaemia reperfusion injury-induced kidney damage. (a–e) Haematoxylin and eosin staining in transplanted (left panels) and non-transplanted kidneys (right panels). (a) 30 min cold ischaemia (CI), (b) 2 h CI without C5aRA and (c) 2 h CI in the presence of C5aRA. Small arrows depict glomeruli and large arrows indicate regions of haemorrhage. Figures are reduced from an original magnification of 20×. (d) Quantification of tissue damage in transplanted (left panel) and non-transplanted (right panel) kidneys. A tissue damage score was determined on a scale of 0–3 (none, mild, moderate and severe) as outlined in Materials and methods, with a maximum possible score of 9 = severest damage being attainable. (e) Spatial distribution of damage in transplanted (left panel) and non-transplanted kidneys (right panel). (f) Relative kidney damage shown as a composite damage score calculating the product of each individual damage score with its corresponding area of damage. All values were determined 72 h post-transplantation (n = 6–12).
Quantitative assessment revealed more pronounced tissue damage in the medulla of the transplanted kidney than in the cortex in all treatment groups (Fig. 2d; left panel). Tissue damage was most severe in kidneys from group B mice, both in transplanted as well as in non-transplanted kidneys. C5aR blockade decreased cortical and medullar damage significantly in the graft, and even more in the non-transplanted kidney (Fig. 2d; right panel).
We further determined the spatial distribution of kidney damage. About 20% or 30% of kidney graft tissue within the cortex or the medulla of group A mice was damaged. In contrast, only 10% of kidney tissue was damaged in the contralateral, non-transplanted kidney (Fig. 2e; right panel). Two hours of CI dramatically increased the magnitude of kidney damage up to ∼ 90% in the transplanted or 50–60% in the non-transplanted kidney, both in the medulla and the cortex. Importantly, kidney damage was reduced to 60–70% in the transplanted kidney of group C mice and was only 10% in the non-transplanted kidney.
To measure more accurately the overall kidney damage in response to IRI, we combined the quantitative assessment (Fig. 2d) and spatial distribution of kidney damage (Fig. 2e) in a score that grades relative kidney damage. As shown in Fig. 2f, the relative kidney damage was: (i) more pronounced in the transplanted kidney compared with the non-transplanted kidney; (ii) higher in the medulla than in the cortex of the transplanted kidney; (iii) most severe in group B kidneys; and (iv) substantially reduced in group C kidneys.
Complement 5a receptor blockade during CI reduces tubular apoptosis
We and others have shown that apoptotic pathways become activated after IRI in rodents [12] and in human renal allografts [24,25]. In agreement with these findings, we observed a low frequency of apoptotic cells (4·3 ± 0·28 apoptotic cells/100 counted tubular cells) in the transplanted kidney of group A mice (Fig. 3a and d). The apoptotic events occurred almost exclusively in tubular epithelial cells but not in glomeruli. Extension of CIT to 2 h was associated with threefold higher numbers of apoptotic tubules (13·2 ± 0·6) in the transplanted kidney (Fig. 3b and d). Importantly, addition of the C5aRA to the UW solution protected from the induction of tubular apoptosis (7·2 ± 0·5 cells; Fig. 3c and d). In contrast to the transplanted kidney, we found only very minor tubular apoptosis in the non-transplanted kidneys and no significant differences between the three treatment groups (Fig. 3a–c and e).
Fig. 3.
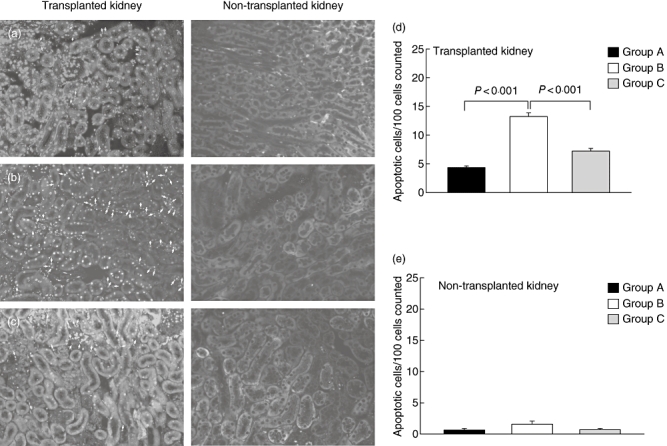
Impact of complement 5a receptor (C5aR) targeting on ischaemia reperfusion injury-induced tubular apoptosis. (a–c) Transferase-mediated dUTP nick-end labelling (TUNEL)-positive tubular epithelial cells in transplanted (left panels) and non-transplanted kidneys (right panels). (a) 30 min cold ischaemia (CI). (b) 2 h CI without C5aRA. (c) 2 h CI in the presence of C5aRA. TUNEL-positive cells in the transplanted kidneys of groups a–c animals are depicted by arrows (left panels). Figures are reduced from an original magnification of 20×. (c, d) Quantification of apoptosis in tubular epithelial cells of transplanted (c) and non-transplanted (d) kidneys (number of TUNEL-positive cells/100 tubular cells in each of five high-power fields). Groups were compared by one way analysis of variance to determine statistical differences between treatment groups (see Material and methods). All values were determined 72 h post transplantation (n = 6–12).
Complement 5a receptor blockade during CI protects from up-regulation of C5aR on tubular cells
Previously, we have shown that 30 min of warm ischaemia results in significant up-regulation of C5aR expression in murine tubular epithelial cells as early as 2 h after reperfusion, which persists for at least 24 h in a murine model of renal IRI [12]. Here, we found no tubular C5aR expression 72 h after CI in group A mice. However, C5aR expression was evident on tissue macrophages (Fig. 4a; left panel). In contrast, we observed intense C5aR staining of tubular epithelial cells in the transplanted kidney after 2 h cold storage (Fig. 4b; left panel), which was almost abrogated following C5aRA treatment (Fig. 4c; left panel). In the contralateral kidneys of mice from all treatment groups, we found C5aR staining of tissue macrophages but not of tubular cells (Fig. 4a–c; right panels). These data suggest that C5aR up-regulation persists for at least 72 h after CI and that C5aR targeting protects from sustained C5aR expression in the transplanted kidney.
Fig. 4.
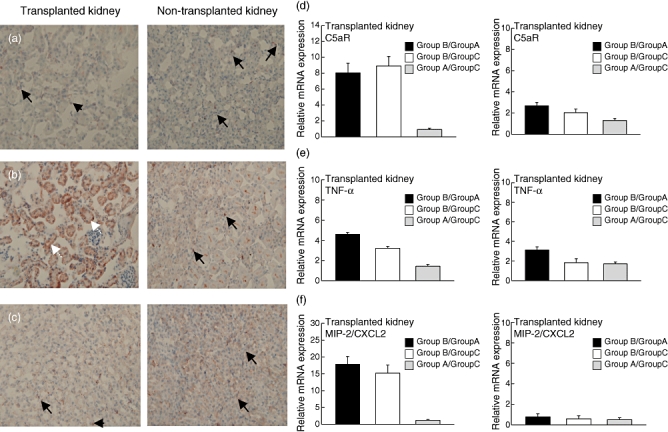
Impact of complement 5a receptor (C5aR) targeting on ischaemia reperfusion injury-induced C5aR expression and tissue inflammation. (a–c) C5aR expression in transplanted (left panels) and non-transplanted kidneys (right panels). (a) 30 min cold ischaemia (CI). Black arrows indicate C5aR positive macrophages; white arrows indicate C5aR positive tubular epithelial cells. (b) 2 h CI without C5aRA. (c) 2 h CI in the presence of C5aRA. Figures are reduced from an original magnification of 20×. (d) C5aR; (e) tumour necrosis factor-α; and (f) macrophage inflammatory protein-2/CXCL2 mRNA expression levels, quantified by real-time polymerase chain reaction in transplanted (left panel) and non-transplanted (right panel) kidneys respectively. The mRNA expression between the indicated treatment groups was compared. All values were determined 72 h post-transplantation (n = 6–12).
In addition to the histological evaluation, we determined C5aR mRNA expression in kidney tissue by quantitative real-time PCR. We found eight- or ninefold higher C5aR expression in grafts from group B mice compared with grafts from group A or group C mice (Fig. 4d; left panel). In non-transplanted kidneys, C5aR expression was only slightly elevated in grafts from group B mice (2·6- or twofold) compared with that from group A and group C mice respectively (Fig. 4d; right panel). C5aR expression levels between group A and group C were indistinguishable.
Decreased kidney inflammation in response to C5aR blockade during CI
Tissue damage following CI was associated with increased kidney inflammation, as evidenced by increased mRNA levels of the proinflammatory cytokine TNF-α and the CXC chemokine MIP-2/CXCL2. We found fourfold or 17-fold higher TNF-α or MIP-2/CXCL2 expression levels in tissues from group B mice than in tissues from group A mice (Fig. 4e and f; left panels). Similarly, TNF-α or MIP-2/CXCL2 expression was 3·2-fold or 15-fold higher in kidneys from group B mice compared with kidneys from group C mice. C5aRA treatment protected from the induction of the proinflammatory response, as evidenced by similar TNF-α and MIP-2/CXCL2 expression levels in kidneys from group A and group C mice. In the non-transplanted kidney, the inflammatory response was much weaker than in thetransplanted kidney and the effect of the C5aRA became less evident (Fig. 4e and f; right panels).
Complement 5a receptor expression is increased in human cadaveric grafts and correlates with CIT, kidney function and apoptosis
Our animal studies demonstrate that C5aR is up-regulated in response to transplantation-associated IRI and that pharmacological targeting of the C5aR during CI has a beneficial impact on early tissue damage, inflammation and apoptosis, eventually improving early graft survival. To assess the role of C5aR in the pathogenesis of transplantation-associated IRI in human kidneys, we investigated a total of 25 paediatric patients, 12 or 13 of whom received allografts from LRD and CAD respectively. Their demographic characteristics, diagnoses and clinical variables are shown in Table 1.
Table 1.
Clinical characteristics of patients with living-related donor (LRD) and cadaveric donor (CAD) kidney transplantation.
| Age | Gender | Ischaemia time | ||||||
|---|---|---|---|---|---|---|---|---|
| (years) | Male | Female | Diseases | (n) | Race | (n) | (min) | |
| LRD | 14·5 ± 0·9 | 6 | 6 | Obstruction | 3 | Caucasian | 3 | |
| Dysplasia | 2 | Hispanic | 4 | |||||
| GN | 3 | African American | 5 | ≤30 | ||||
| FSGS | 4 | |||||||
| CAD | 13·5 ± 0·9 | 6 | 7 | Obstruction | 4 | Caucasian | 3 | |
| Dysplasia | 4 | Hispanic | 4 | |||||
| GN | 3 | African American | 6 | 1395 ± 77 | ||||
| FSGS | 2 | |||||||
FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis.
All CAD samples showed typical histological evidence of IRI, including flattening of proximal tubule cells, loss of brush border, tubular cast formation and occasional necrotic cells, as described previously [24]. C5aR was up-regulated in this group compared with the LRD in all tissues examined (Fig. 5a and b). Quantitative determination of C5aR expression revealed significantly higher values in the CAD group than in the LRD group (Fig. 5c). As expected, we found a strong positive correlation between CIT and sCr levels (Fig. 5d). Importantly, C5aR expression levels in the CAD group showed significant positive correlations with peak sCr (Fig. 5e), duration of CIT (Fig. 5f) and the frequency of apoptotic cells (Fig. 5g). These data suggest that CIT drives the up-regulation of C5aR expression in human kidney tissue which, in turn, promotes kidney damage and tubular apoptosis, leading eventually to increased sCr.
Fig. 5.
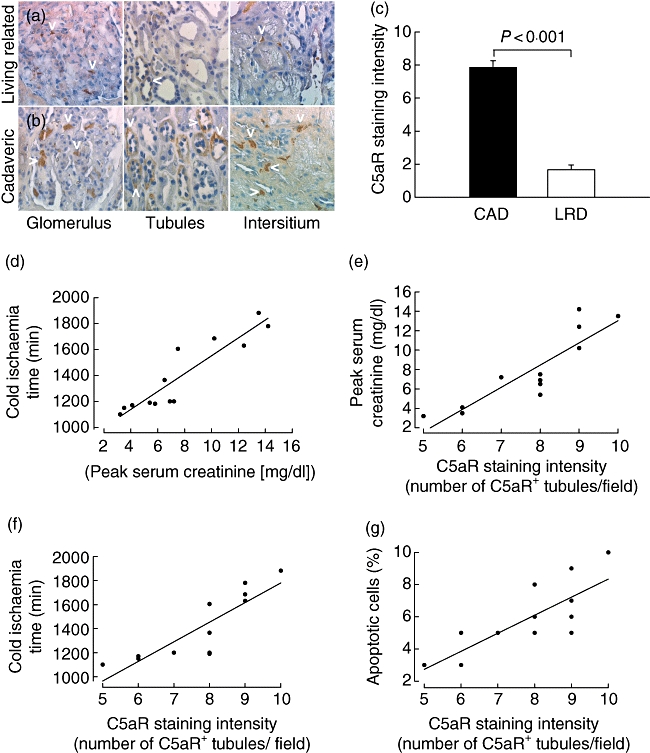
Complement 5a receptor (C5aR) expression in cadaveric donor (CAD) and living-related donor (LRD) and its correlation with cold ischaemia time, kidney function and tubular cell apoptosis. (a, b) C5aR expression in glomeruli, tubules and the interstitium of human allografts from LRD (a, upper panels) or CAD (b, lower panels). C5aR staining is depicted by the white arrowheads in each panel. (c) Quantitative evaluation of C5aR expression in grafts from CAD (n = 13) and LRD (n = 12). Groups were compared by analysis of variance to determine statistical differences between treatment groups (see Material and methods). (d) Correlation between cold ischaemia time (CIT) and peak serum creatinine (sCr) levels (r = 0·97, P < 0·001). (e–g) Correlations between C5aR staining intensity (number of C5aR positive tubules/field) and peak sCr levels (r = 0·96, P < 0·001) (e), duration of CIT (r = 0·82, P < 0·001) (f) and the frequency of apoptotic cells in the allograft (r = 0·78, P = 0·001) (g).
Discussion
Ischaemia and reinstitution of blood flow in ischaemically damaged kidneys activate a complex sequence of eventsthat sustain renal injury and play a pivotal role in thedevelopment of DGF, resulting in post-transplantation oliguria in 20–40% of primary cadaveric renal transplants in the US [26,27]. DGF has been linked to poorer prognosis, resulting in either acute rejection or significantly decreased 1- and 5-year graft survival [28]. Although the pathogenesis of IRI is complex and understood incompletely, several animal studies have demonstrated convincingly that local activation of the complement system is critical to the development of IRI (reviewed in [6,7]).
Despite the significant advances that have been made in understanding the mechanisms underlying IRI in animal models, little progress has been made in therapeutic approaches over the past decades. Haemodialysis is used currently as a supportive therapy as no other, more effective treatment is available [29,30].
To minimize the effects of organ damage and IRI, organ donation requires both in situ flush with a cold preservation solution and hypothermic storage. UW solution is the standard solution used for abdominal organ transplantation, including the kidney [17]. Importantly, the preservation effects of even the market leader UW is anything but optimal, leading to DGF rates of 20–40%. A meta-analysis examining the modes of preservation-linked DGF and graft half-life concluded that DGF could contribute to a 20% reduction in 10-year graft survival compared with that observed with immediately functioning renal allografts [30]. Therefore, the number of functioning graft years lost worldwide as a consequence of poor organ preservation appears to be significant. Clinical and experimental studies suggest that improvements in organ preservation will have significant benefits for long-term outcomes. Hence the optimization of organ preservation may permit better use of the scarce resource of donor organs.
In order to optimize organ preservation, we have targeted the C5aR within the kidney tissue by supplementing UW solution with C5aRA. This molecule functions as a competitive C5aRA for human and mouse C5aR and C5L2 [21]. The significant therapeutic potential of C5aRA has been demonstrated in a variety of diseases models, such as renal [12] and intestinal [31] IRI, immune complex disease [31,32], experimental allergic asthma [33,34] and infection with intracellular parasites [23].
Our data show that 30 min of warm ischaemia combined with 2 h of CI induces substantial cortical and medullary tissue damage involving glomeruli and tubules, leading eventually to graft loss. Blockade of C5aR during CI reduces tissue damage significantly in the graft as well as remote injury in the non-transplanted kidney, resulting ultimately in a significantly improved graft survival rate. Mechanistically, C5aR expression has been identified on human and mouse tissue macrophages [35,36] as well as on human proximal tubular and glomerular mesangial cells [35,37,38] of naive kidneys. After renal IRI, C5aR expression on tubular and mesangial cells is up-regulated up to 24 h after the insult [12]. In agreement with our previous data, we found that 2 h CI resulted in C5aR up-regulation on tubular cells, which persisted for 72 h. Targeting of the C5aR during CI prevented the sustained C5aR tubular expression. At first glance, this effect is surprising as the C5aRA was administered only locally during CI. However, C5aR expression is regulated positively by cytokines such as interleukin (IL)-6 [39] which, in turn, can be induced by C5aR activation on tissue macrophages [40]. Thus, local C5aR blockade at the time of reperfusion might be critical to prevent C5a-induced release of proinflammatory cytokines and chemokines, which would otherwise promote a proinflammatory amplification loop. In support of this view, we found decreased TNF-α and MIP-2 expression levels in grafts of mice that had been treated with the C5aRA. In addition to tissue macrophages, hypoxia-stressed proximal tubular epithelial cells and glomerular mesangial cells are an important source of TNF-α. Importantly, TNF-α or MIP-2 blockade in vivo protects from the development of renal IRI-induced tissue damage and apoptotic/necrotic death of tubular cells [41,42]. These data suggest that early C5aR blockade suppresses the development of kidney damage and dysfunction by counterbalancing IRI-induced development of tissue inflammation and apoptosis/necrosis which, in concert, promote DGF.
The relevance of C5aR blockade as a potential therapeutic concept in human kidney transplantation is emphasized by our data, showing that C5aR expression is up-regulated soon after CI in grafts from CAD and that this up-regulation is associated strongly with CIT, impaired kidney function and the frequency of apoptotic tubular epithelial cells. These data indicate that the mechanisms leading to ARF and graft loss in murine models of kidney transplantation and in human paediatric patients are similar, at least with regard to C5a-induced graft pathology.
The concept of blocking C5aR and C5L2 during transplantation-associated CI has not been explored before. Previous concepts have focused on complement regulatory molecules such as the complement receptor 1-related protein y (Crry) [43], C3-specific small interfering RNA [44] or a membrane-localizing complement regulator derived from human complement receptor type 1 (APT070) [45,46]. While Crry failed to protect kidneys from IRI-induced injury, systemic delivery of anti-C3 siRNA reduced IRI-mediated renal injury in mice. APT070 was used in a rat model of syngeneic kidney transplantation as an additive to the preservation solution. Importantly, this treatment was associated with less tubular damage and improved renal function, leading eventually to increased graft survival (from 26·3% to 63·6%), the extent of which was similar to what we have found in our study (Fig. 1). APT070 interacts with C3b and C4b, leading to dissociation of both molecules from C3 or C5 convertases and acts as a co-factor for factor I to degrade C3b and C4b. Thus APT070 promotes broad inhibition of the complement system, whereas C5aRA specifically blocks the C5aR pathway, leaving major functions of the system such as opsonization, lysis and clearance of apoptotic cells intact.
In addition to the impact on inflammation and apoptosis, recent reports imply that C5aR blockade will also affect the induction of adaptive immune responses following allotransplantation. Previously, we have shown that C5aRA inhibits microbial-induced production of IL-12 from human monocytes [33]. Extending this finding, Lalli et al. recently found that allospecific T helper tpe 1 differentiation is impaired substantially when antigen-presenting cells lack the C5aR [47]. Collectively, these data suggest that C5aR targeting in renal transplantation may not only protect from early inflammation induced by innate immune mechanisms but also from the development of allospecific adaptive immune responses promoting allograft rejection.
In conclusion, we demonstrate that administration of a C5aR/C5L2 inhibitor to UW solution reduces IRI-induced kidney damage and tubular cell apoptosis as well as sustained up-regulation of C5aR in tubular epithelial cells in a model of syngeneic kidney transplantation. Thus C5aR targeting during CI might prove useful to protect from the development of transplantation-associated DGF.
Acknowledgments
We gratefully acknowledge the outstanding technical advice provided by Song Rong and Faikah Güler (Department of Nephrology, Medical School Hannover, Germany). This work was funded by Translational Research Initiative of Cincinnati Children's Hospital Medical Center.
References
- 1.Najarian JS, Gillingham KJ, Sutherland DE, Reinsmoen NL, Payne WD, Matas AJ. The impact of the quality of initial graft function on cadaver kidney transplants. Transplantation. 1994;57:812–16. doi: 10.1097/00007890-199403270-00007. [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–74. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Lu CY, Penfield JG, Kielar ML, Vazquez MA, Jeyarajah DR. Hypothesis: is renal allograft rejection initiated by the response to injury sustained during the transplant process? Kidney Int. 1999;55:2157–68. doi: 10.1046/j.1523-1755.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–78. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 5.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–20. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 6.Sacks SH, Chowdhury P, Zhou W. Role of the complement system in rejection. Curr Opin Immunol. 2003;15:487–92. doi: 10.1016/s0952-7915(03)00100-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W, Medof ME, Heeger PS, Sacks S. Graft-derived complement as a mediator of transplant injury. Curr Opin Immunol. 2007;19:569–76. doi: 10.1016/j.coi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–15. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- 9.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170:1517–23. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- 10.de Vries B, Walter SJ, Peutz-Kootstra CJ, Wolfs TG, van Heurn LW, Buurman WA. The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia–reperfusion injury. Am J Pathol. 2004;165:1677–88. doi: 10.1016/S0002-9440(10)63424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller-Kristensen M, Wang W, Ruseva M, et al. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol. 2005;61:426–34. doi: 10.1111/j.1365-3083.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- 12.De Vries B, Köhl J, Leclercq WK, et al. Complement factor C5a mediates renal ischemia–reperfusion injury independent from neutrophils. J Immunol. 2003;170:3883–9. doi: 10.4049/jimmunol.170.7.3883. [DOI] [PubMed] [Google Scholar]
- 13.Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003;63:134–42. doi: 10.1046/j.1523-1755.2003.00737.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Farrar CA, Abe K, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363–71. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K, Sacks SH, Zhou W. The relative importance of local and systemic complement production in ischaemia, transplantation and other pathologies. Mol Immunol. 2007;44:3866–74. doi: 10.1016/j.molimm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Hart ML, Walsh MC, Stahl GL. Initiation of complement activation following oxidative stress. In vitro and in vivo observations. Mol Immunol. 2004;41:165–71. doi: 10.1016/j.molimm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–47. doi: 10.1146/annurev.med.46.1.235. [DOI] [PubMed] [Google Scholar]
- 18.Opelz G, Dohler B. Multicenter analysis of kidney preservation. Transplantation. 2007;83:247–53. doi: 10.1097/01.tp.0000251781.36117.27. [DOI] [PubMed] [Google Scholar]
- 19.Kootstra G, van HE. Non-heartbeating donation of kidneys for transplantation. Nat Clin Pract Nephrol. 2007;3:154–63. doi: 10.1038/ncpneph0426. [DOI] [PubMed] [Google Scholar]
- 20.Delmonico FL, Dew MA. Living donor kidney transplantation in a global environment. Kidney Int. 2007;71:608–14. doi: 10.1038/sj.ki.5002125. [DOI] [PubMed] [Google Scholar]
- 21.Otto M, Hawlisch H, Monk PN, et al. C5a mutants are potent antagonists of the C5a receptor (CD88) and of C5L2: position 69 is the locus that determines agonism or antagonism. J Biol Chem. 2004;279:142–51. doi: 10.1074/jbc.M310078200. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler E, Gueler F, Rong S, et al. CCL19-IgG prevents allograft rejection by impairment of immune cell trafficking. J Am Soc Nephrol. 2006;17:2521–32. doi: 10.1681/ASN.2005070782. [DOI] [PubMed] [Google Scholar]
- 23.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Köhl J. C5a negatively regulates Toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–26. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, et al. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemia reperfusion injury. Transplantation. 2003;76:50–4. doi: 10.1097/01.TP.0000069835.95442.9F. [DOI] [PubMed] [Google Scholar]
- 25.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608–27. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 26.Koning OH, Ploeg RJ, Van Bockel JH, et al. Risk factors for delayed graft function in cadaveric kidney transplantation: a prospective study of renal function and graft survival after preservation with University of Wisconsin solution in multi-organ donors. Transplantation. 1997;63:1620–8. doi: 10.1097/00007890-199706150-00015. European Multicenter Study Group. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqi N, McBride MA, Hariharan S. Similar risk profiles for post-transplant renal dysfunction and long-term graft failure: UNOS/OPTN database analysis. Kidney Int. 2004;65:1906–13. doi: 10.1111/j.1523-1755.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 28.Daly PJ, Power RE, Healy DA, Hickey DP, Fitzpatrick JM, Watson RW. Delayed graft function: a dilemma in renal transplantation. BJU Int. 2005;96:498–501. doi: 10.1111/j.1464-410X.2005.05673.x. [DOI] [PubMed] [Google Scholar]
- 29.Andreoli SP. Acute renal failure. Curr Opin Pediatr. 2002;14:183–8. doi: 10.1097/00008480-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–27. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 31.Heller T, Hennecke M, Baumann U, et al. Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J Immunol. 1999;163:985–94. [PubMed] [Google Scholar]
- 32.Baumann U, Köhl J, Tschernig T, et al. A codominant role of Fc gamma RI/III and C5aR in the reverse Arthus reaction. J Immunol. 2000;164:1065–70. doi: 10.4049/jimmunol.164.2.1065. [DOI] [PubMed] [Google Scholar]
- 33.Karp CL, Grupe A, Schadt E, et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol. 2000;1:221–6. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- 34.Köhl J, Baelder R, Lewkowich IP, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116:783–96. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fayyazi A, Scheel O, Werfel T, et al. The C5a receptor is expressed in normal renal proximal tubular but not in normal pulmonary or hepatic epithelial cells. Immunology. 2000;99:38–45. doi: 10.1046/j.1365-2567.2000.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo RF, Ward PA. Role of c5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 37.Zahedi R, Braun M, Wetsel RA, et al. The C5a receptor is expressed by human renal proximal tubular epithelial cells. Clin Exp Immunol. 2000;121:226–33. doi: 10.1046/j.1365-2249.2000.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe K, Miyazaki M, Koji T, et al. Enhanced expression of complement C5a receptor mRNA in human diseased kidney assessed by in situ hybridization. Kidney Int. 2001;60:137–46. doi: 10.1046/j.1523-1755.2001.00780.x. [DOI] [PubMed] [Google Scholar]
- 39.Riedemann NC, Neff TA, Guo RF, et al. Protective effects of IL-6 blockade in sepsis are linked to reduced c5a receptor expression. J Immunol. 2003;170:503–7. doi: 10.4049/jimmunol.170.1.503. [DOI] [PubMed] [Google Scholar]
- 40.Höpken UE, Lu B, Gerard NP, Gerard C. Impaired inflammatory responses in the reverse Arthus reaction through genetic deletion of the C5a receptor. J Exp Med. 1997;186:749–56. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daemen MA, van de V, Heineman E, Buurman WA. Involvement of endogenous interleukin-10 and tumor necrosis factor-alpha in renal ischemia–reperfusion injury. Transplantation. 1999;67:792–800. doi: 10.1097/00007890-199903270-00003. [DOI] [PubMed] [Google Scholar]
- 42.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol. 2001;159:2137–45. doi: 10.1016/s0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park P, Haas M, Cunningham PN, et al. Inhibiting the complement system does not reduce injury in renal ischemia reperfusion. J Am Soc Nephrol. 2001;12:1383–90. doi: 10.1681/ASN.V1271383. [DOI] [PubMed] [Google Scholar]
- 44.Zheng X, Feng B, Chen G, et al. Preventing renal ischemia–reperfusion injury using small interfering RNA by targeting complement 3 gene. Am J Transplant. 2006;6:2099–108. doi: 10.1111/j.1600-6143.2006.01427.x. [DOI] [PubMed] [Google Scholar]
- 45.Pratt JR, Jones ME, Dong J, et al. Nontransgenic hyperexpression of a complement regulator in donor kidney modulates transplant ischemia/reperfusion damage, acute rejection, and chronic nephropathy. Am J Pathol. 2003;163:1457–65. doi: 10.1016/S0002-9440(10)63503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel H, Smith RA, Sacks SH, Zhou W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J Am Soc Nephrol. 2006;17:1102–11. doi: 10.1681/ASN.2005101116. [DOI] [PubMed] [Google Scholar]
- 47.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-{gamma}-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179:5793–802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]


