Abstract
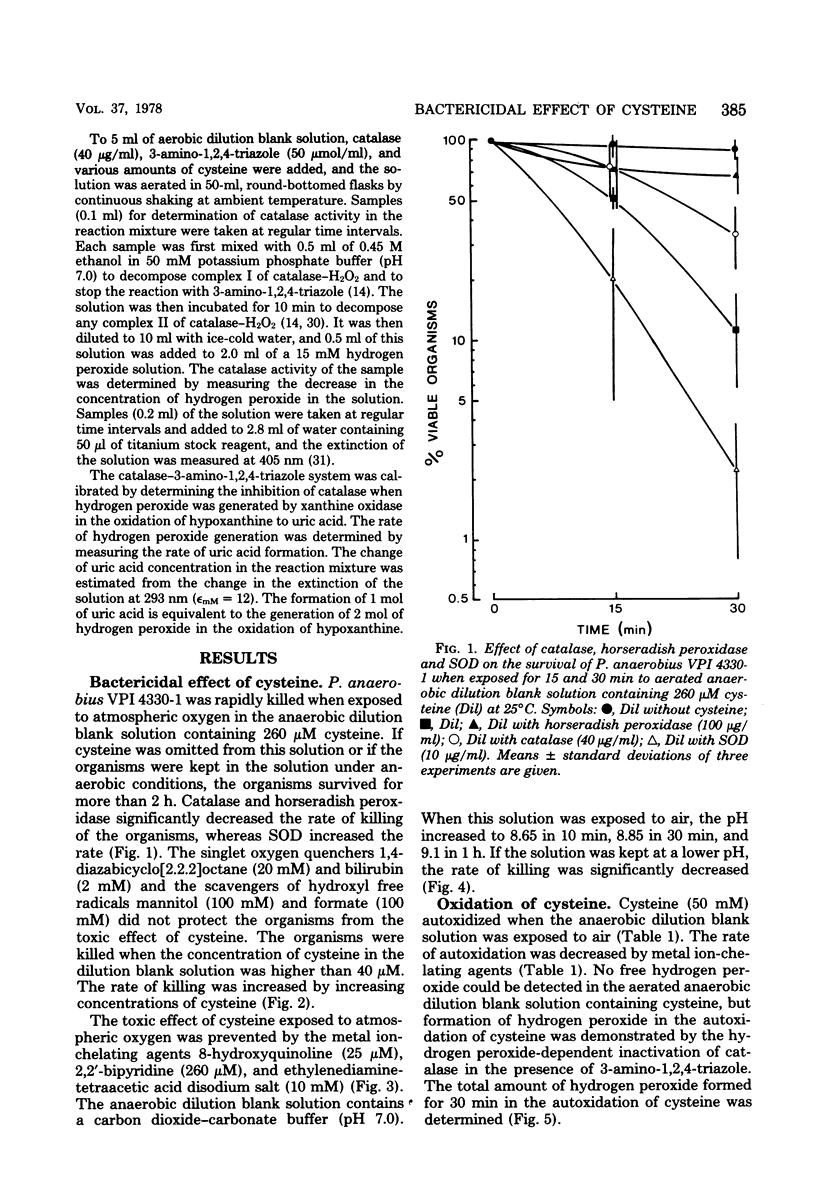
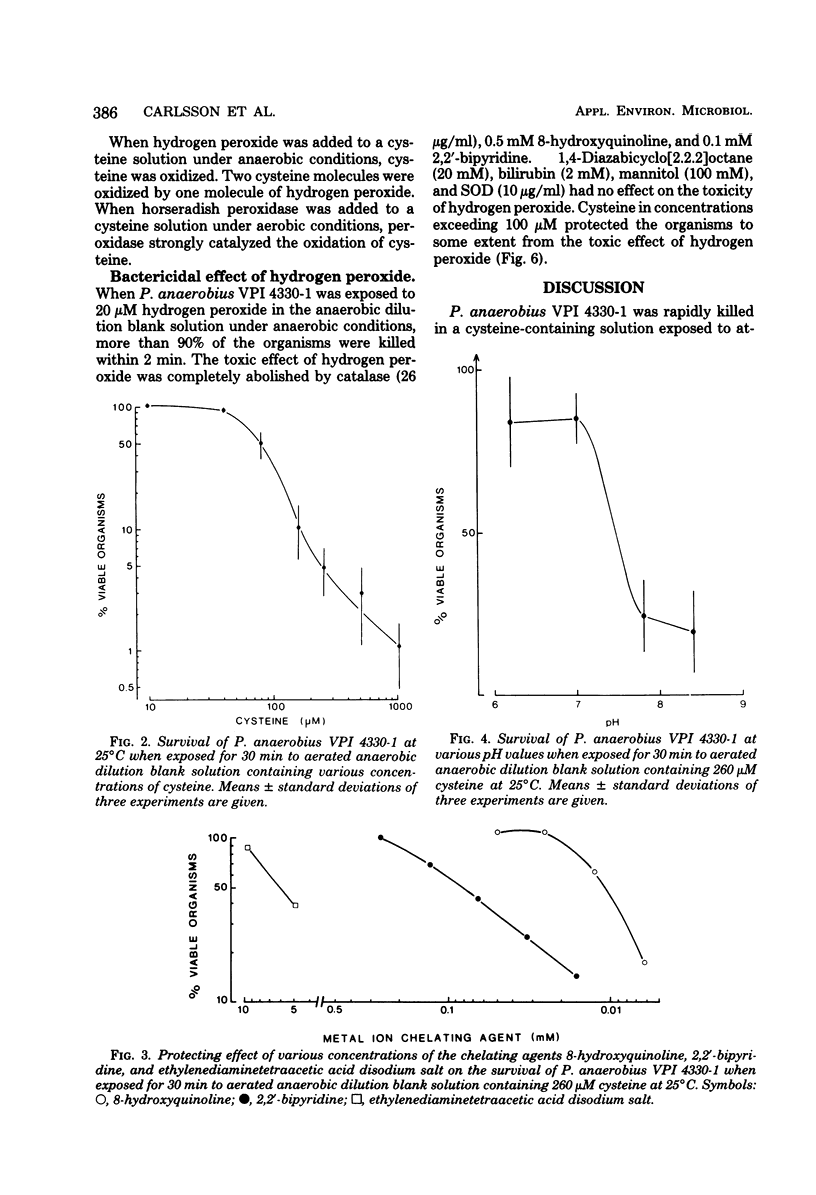
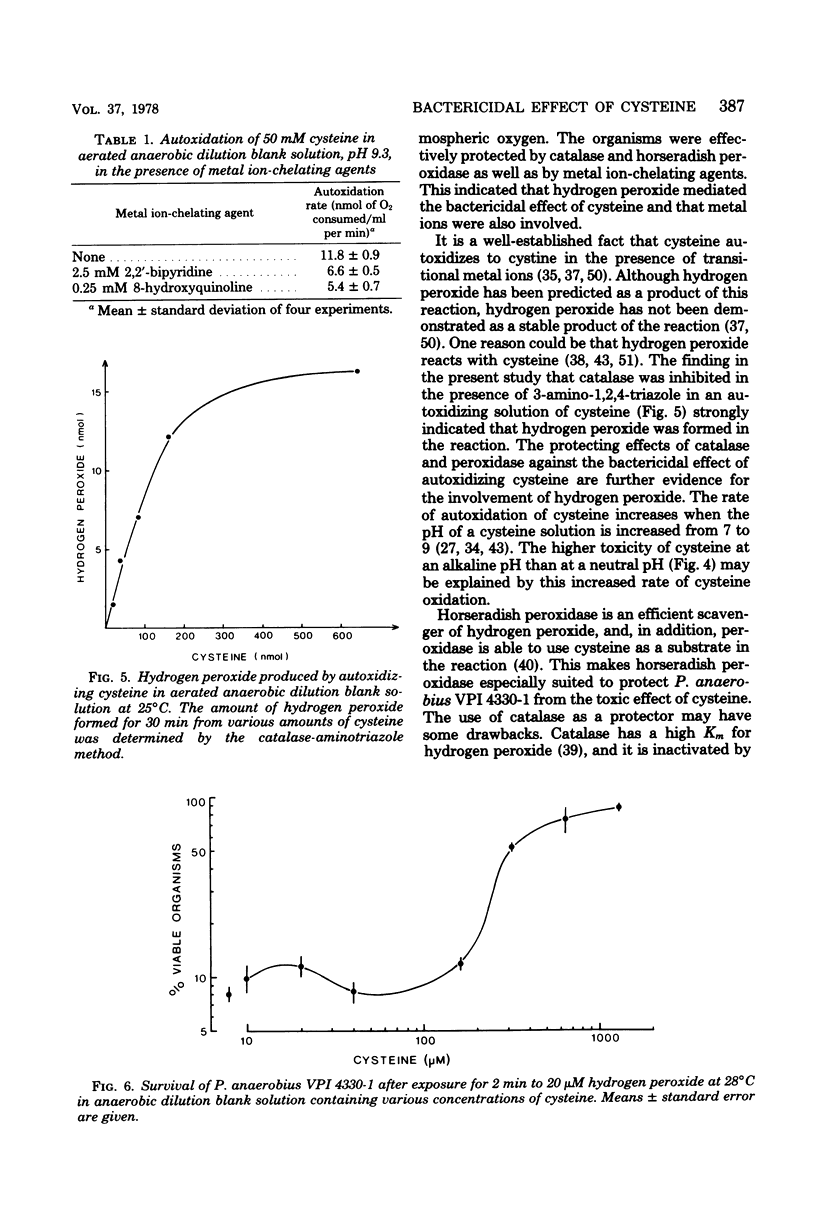
Peptostreptococcus anaerobius VPI 4330-1 was exposed to atmospheric oxygen in a dilution bland (0.2% gelatin, salts, resazurin) solution. The organisms were rapidly killed when the solution contained cysteine. The organisms were effectively protected by catalase and horseradish peroxidase as well as by the metal ion-chelating agents 8-hydroxyquinoline and 2,2'-bipyridine. Superoxide dismutase increased the rate of killing of the organisms, whereas singlet oxygen quenchers and scavengers of hydroxyl free radicals did not protect the organisms from the toxic effect of cysteine. Hydrogen peroxide was formed when cysteine was exposed to oxygen in the dilution blank solution, and the reaction was inhibited by metal ion-chelating agents. The organisms were rapidly killed by 20 microM hydrogen peroxide in anaerobic dilution blank solution. The toxic effect of hydrogen peroxide in anaerobic dilution blank solution. The toxic effect of hydrogen peroxide was completely abolished by catalase and metal ion-chelating agents. These results indicated that hydrogen peroxide was formed in the dilution blank solution in a metal ion-catalyzed autoxidation of cysteine and that hydrogen peroxide was toxic to P. anaerobius VPI 4330-1 in a reaction also catalyzed by metal ions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A. Quantitative studies of the avidity of naturally occurring substances for trace metals. II. Amino-acids having three ionizing groups. Biochem J. 1952 Mar;50(5):690–697. doi: 10.1042/bj0500690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER N. M. Catalase inhibition by normal and neoplastic tissue extracts. J Biol Chem. 1957 Aug;227(2):975–985. [PubMed] [Google Scholar]
- Ahmad M., Srivastava B. S., Agarwala S. C. Effect of incubation media on the recovery of Escherichia coli K12 heated at 52 degrees C. J Gen Microbiol. 1978 Jul;107(1):37–44. doi: 10.1099/00221287-107-1-37. [DOI] [PubMed] [Google Scholar]
- Allen E. H., Hussey G. G. Inhibition of the growth of Helminthosporium carbonum by L-cysteine. Can J Microbiol. 1971 Jan;17(1):101–103. doi: 10.1139/m71-017. [DOI] [PubMed] [Google Scholar]
- Amsden A. B., Small D. K., Gomez R. F. Complex medium toxicity to some DNA repair-deficient strains of Salmonella typhimurium. Can J Microbiol. 1977 Oct;23(10):1494–1496. doi: 10.1139/m77-221. [DOI] [PubMed] [Google Scholar]
- Beuchat L. R. Injury and repair of gram-negative bacteria, with special consideration of the involvement of the cytoplasmic membrane. Adv Appl Microbiol. 1978;23:219–243. doi: 10.1016/s0065-2164(08)70071-6. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswaran C., Sreenivasan A., Rege D. V. Effect of cysteine on respiration and catalyse synthesis by Saccharomyces cerevisiae. Biochem J. 1964 Sep;92(3):504–508. doi: 10.1042/bj0920504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer D. G., Martin S. E., Ordal Z. J. Beneficial effects of catalase or pyruvate in a most-probable-number technique for the detection of Staphylococcus aureus. Appl Environ Microbiol. 1977 Dec;34(6):797–800. doi: 10.1128/aem.34.6.797-800.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A., Ashwood-Smith M. J., Munson R. J. Correlation of bacterial sensitivities to ionizing radiation and mild heating. J Gen Microbiol. 1969 Sep;58(1):115–124. doi: 10.1099/00221287-58-1-115. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Frölander F., Sundquist G. Oxygen tolerance of anaerobic bacteria isolated from necrotic dental pulps. Acta Odontol Scand. 1977;35(3):139–145. doi: 10.3109/00016357709056002. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Nyberg G., Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol. 1978 Aug;36(2):223–229. doi: 10.1128/aem.36.2.223-229.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Catalase-aminotriazole method for measuring secretion of hydrogen peroxide by microorganisms. J Bacteriol. 1969 May;98(2):543–546. doi: 10.1128/jb.98.2.543-546.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Pecci L., Pensa B., Cannella C. Hydrogen peroxide involvement in the rhodanese inactivation by dithiothreitol. Biochem Biophys Res Commun. 1977 Sep 23;78(2):596–603. doi: 10.1016/0006-291x(77)90221-2. [DOI] [PubMed] [Google Scholar]
- DALE W. M., RUSSELL C. A study of the irradiation of catalase by ionizing radiations in the presence of cysteine, cystine and glutathione. Biochem J. 1956 Jan;62(1):50–57. doi: 10.1042/bj0620050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers R. S., Martin S. E., Brewer D. G., Ordal Z. J. Catalase and enumeration of stressed Staphylococcus aureus cells. Appl Environ Microbiol. 1977 May;33(5):1112–1117. doi: 10.1128/aem.33.5.1112-1117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey H. E., Pollard E. C. The action of gamma-ray-irradiated medium on bacteria: relation to the electron transport system. Radiat Res. 1968 Oct;36(1):59–67. [PubMed] [Google Scholar]
- Frölander F., Carlsson J. Bactericidal effect of anaerobic broth exposed to atmospheric oxygen tested on Peptostreptococcus anaerobius. J Clin Microbiol. 1977 Aug;6(2):117–123. doi: 10.1128/jcm.6.2.117-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R. F., Sinskey A. J. Effect of aeration on minimal medium recovery of heated Salmonella typhimurium. J Bacteriol. 1975 Apr;122(1):106–109. doi: 10.1128/jb.122.1.106-109.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Patel L. The role of functional sulfhydryl groups in active transport in Escherichia coli membrane vesicles. Biochemistry. 1978 May 2;17(9):1640–1646. doi: 10.1021/bi00602a010. [DOI] [PubMed] [Google Scholar]
- Kari C., Nagy Z., Kovács P., Hernádi F. Mechanism of the growth inhibitory effect of cysteine on Escherichia coli. J Gen Microbiol. 1971 Nov;68(3):349–356. doi: 10.1099/00221287-68-3-349. [DOI] [PubMed] [Google Scholar]
- Keller K. M., Pollard E. C. Action of hydrogen peroxide on degradation of DNA after irradiation in Escherichia coli. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 May;31(5):407–413. doi: 10.1080/09553007714550511. [DOI] [PubMed] [Google Scholar]
- Kobashi K. Catalytic oxidation of sulfhydryl groups by o-phenanthroline copper complex. Biochim Biophys Acta. 1968 May;158(2):239–245. doi: 10.1016/0304-4165(68)90136-0. [DOI] [PubMed] [Google Scholar]
- Little C., O'Brien P. J. Mechanism of peroxide-inactivation of the sulphydryl enzyme glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1969 Oct;10(3):533–538. doi: 10.1111/j.1432-1033.1969.tb00721.x. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSART L., HORENS J. L'assimilation d'azote aminé par les levures. Enzymologia. 1953 Feb;15(6):359–361. [PubMed] [Google Scholar]
- Martin S. E., Flowers R. S., Ordal Z. J. Catalase: its effect on microbial enumeration. Appl Environ Microbiol. 1976 Nov;32(5):731–734. doi: 10.1128/aem.32.5.731-734.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- OGURA Y. Catalase activity at high concentration of hydrogen peroxide. Arch Biochem Biophys. 1955 Aug;57(2):288–300. doi: 10.1016/0003-9861(55)90291-5. [DOI] [PubMed] [Google Scholar]
- Olsen J., Davis L. The oxidation of dithiothreitol by peroxidases and oxygen. Biochim Biophys Acta. 1976 Sep 14;445(2):324–329. doi: 10.1016/0005-2744(76)90086-3. [DOI] [PubMed] [Google Scholar]
- Pauling C., Beck L. A. Role of DNA ligase in the repair of single strand breaks induced in DNA by mild heating of Escherichia coli. J Gen Microbiol. 1975 Mar;87(1):181–184. doi: 10.1099/00221287-87-1-181. [DOI] [PubMed] [Google Scholar]
- Pierson M. D., Gomez R. F., Martin S. E. The involvement of nucleic acids in bacterial injury. Adv Appl Microbiol. 1978;23:263–285. doi: 10.1016/s0065-2164(08)70073-x. [DOI] [PubMed] [Google Scholar]
- Pirie N. W. The oxidation of sulphydryl compounds by hydrogen peroxide: Catalysis of oxidation of cysteine and glutathione by iron and copper. Biochem J. 1931;25(5):1565–1579. doi: 10.1042/bj0251565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman M. K., Aris B., El Derea H. B. The effect of compounds which degrade hydrogen peroxide on the enumeration of heat-stressed cells of Salmonella senftenberg. Can J Microbiol. 1978 Jul;24(7):883–885. doi: 10.1139/m78-146. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., Carr H. S., Morgan C. Unusual growth properties of a bacterial strain lacking DNA polymerase. Biochem Biophys Res Commun. 1971 Aug 6;44(3):546–549. doi: 10.1016/s0006-291x(71)80117-1. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Evidence for indirect production of DNA strand scissions during mild heating of Escherichia coli. J Gen Microbiol. 1972 Jun;71(1):191–193. doi: 10.1099/00221287-71-1-191. [DOI] [PubMed] [Google Scholar]
- Taylor J. E., Yan J. F., Wang J. L. The iron(3)-catalyzed oxidation of cysteine by molecular oxygen in the aqueous phase. An example of a two-thirds-order reaction. J Am Chem Soc. 1966 Apr 20;88(8):1663–1667. doi: 10.1021/ja00960a016. [DOI] [PubMed] [Google Scholar]
- Villarejo M., Westley J. Sulfur metabolism of Bacillus subtilis. Biochim Biophys Acta. 1966 Mar 28;117(1):209–216. doi: 10.1016/0304-4165(66)90168-1. [DOI] [PubMed] [Google Scholar]
- Woodcock E., Grigg G. W. Repair of thermally induced DNA breakage in Escherichia coli. Nat New Biol. 1972 May 17;237(72):76–79. doi: 10.1038/newbio237076a0. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


