Abstract
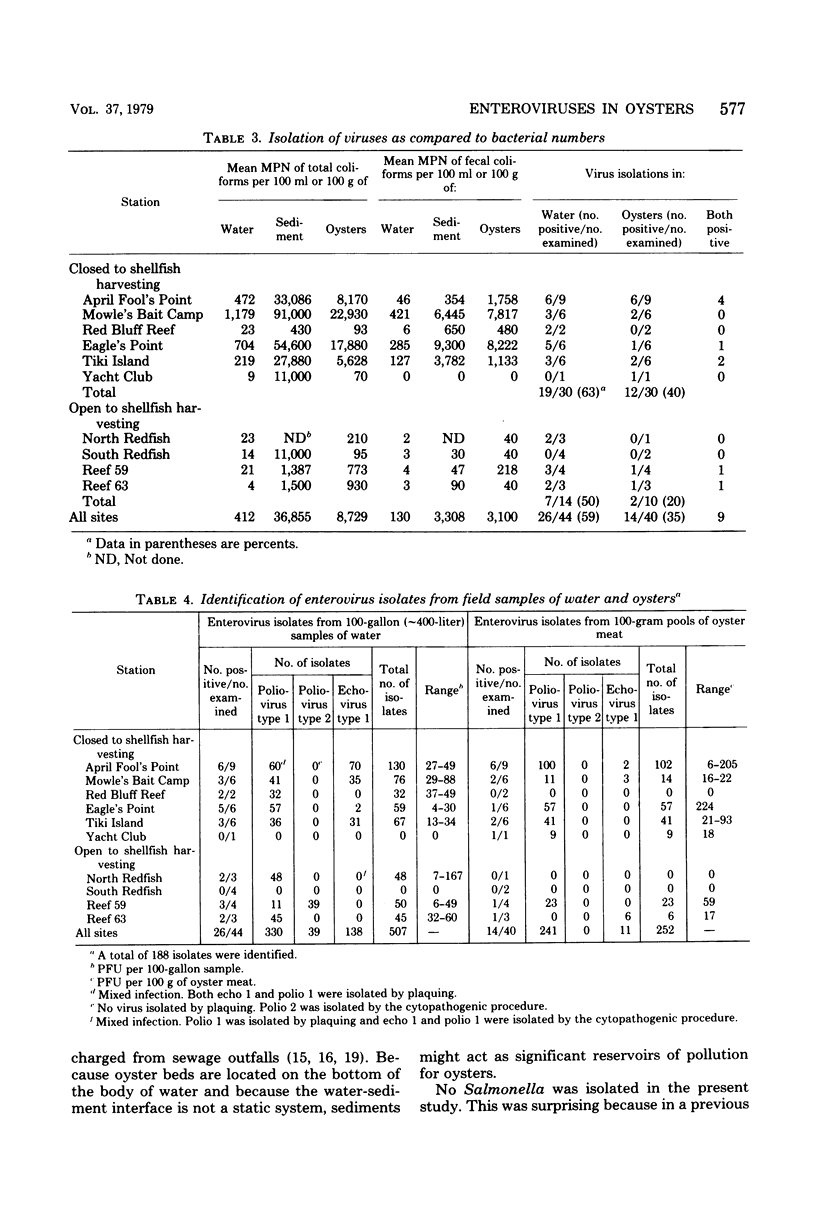
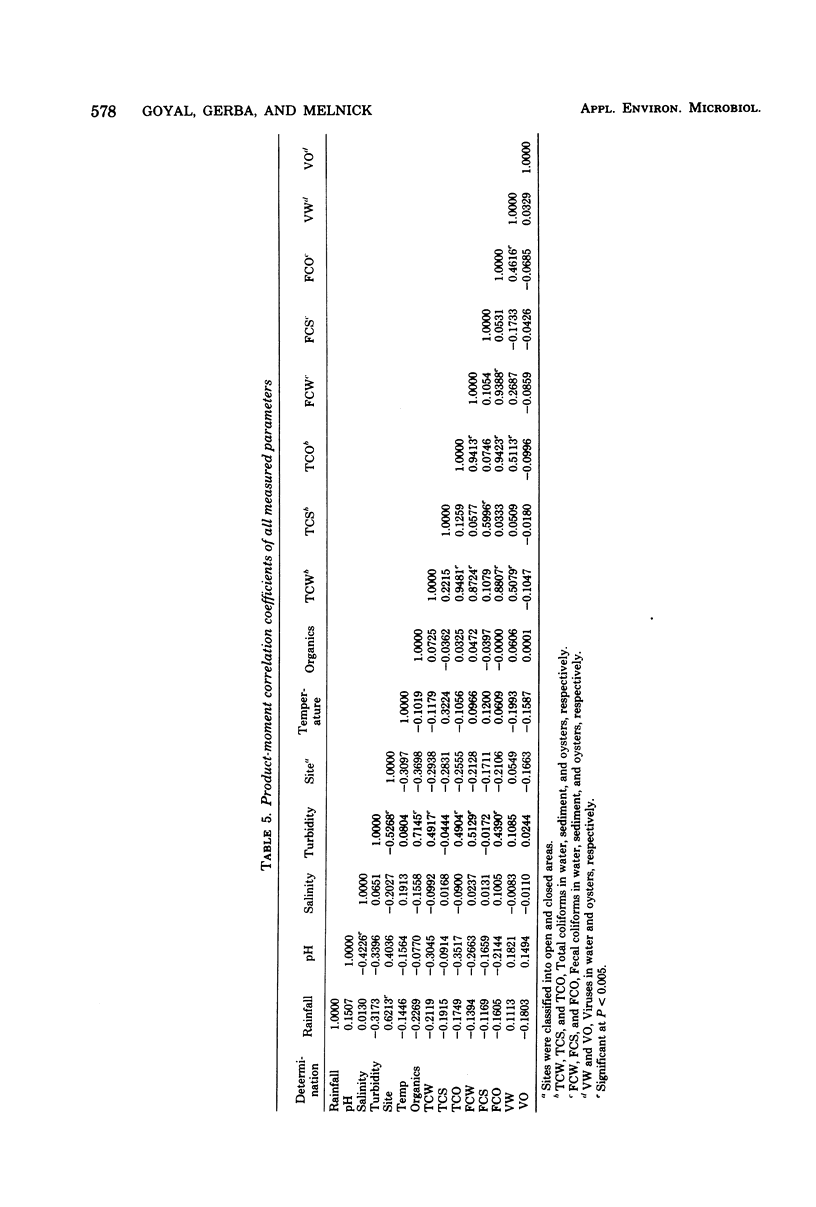
The presence of enteroviruses in oysters and oyster-harvesting waters of the Texas Gulf coast was monitored over a period of 10 months. Viruses were detected in water and oyster samples obtained from areas both open and closed to shellfish harvesting. Viruses were detected periodically in waters that met current bacteriological standards for shellfish harvesting. No significant statistical relationship was demonstrated between virus concentration in oysters and the bacteriological and physiochemical quality of water and shellfish. Viruses in water were, however, moderately correlated with total coliforms in water and oysters and with fecal coliforms in oysters. Total coliforms in water were realted to total coliforms in sediment were related only to total coliforms in sediment. Among the physiochemical characteristics of water, turbidity was related statistically to the organic matter content of water and to fecal coliforms in water. There was a marked effect of rainfall on the bacteriological quality of water. Of a total of 44 water samples, 26 yielded virus in concentrations from 4 to 167 plaque-forming units per 100-gallon (ca. 378.5-liter) sample. Of a total of 40 pools of 10 to 12 oysters each, virus was found in 14 pools at a concentration of 6 to 224 plaque-forming units per 100 g of oyster meat. On five occasions, virus was found in water samples when no virus could be detected in oysters harvested from the same sites. This study indicates that current bacteriological standards for determining the safety of shellfish and shellfish-growing waters do no reflect the occurrence of enteroviruses.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendinelli M., Ruschi A. Isolation of human enterovirus from mussels. Appl Microbiol. 1969 Sep;18(3):531–532. doi: 10.1128/am.18.3.531-532.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney J. F., Carty C. E., Colwell R. R. Seasonal occurrence and distribution of microbial indicators and pathogens in the Rhode River of Chesapeake Bay. Appl Microbiol. 1975 Nov;30(5):771–780. doi: 10.1128/am.30.5.771-780.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliver D. O. Food-associated viruses. Health Lab Sci. 1967 Oct;4(4):213–221. [PubMed] [Google Scholar]
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- De Flora S., De Renzi G. P., Badolati G. Detection of animal viruses in coastal seawater and sediments. Appl Microbiol. 1975 Sep;30(3):472–475. doi: 10.1128/am.30.3.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo R., Wiczynski L., Daley M., Miranda F. Preliminary observations on the uptake poliovirus by West Coast shore crabs. Appl Microbiol. 1972 Jan;23(1):170–171. doi: 10.1128/am.23.1.170-171.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Wallis C., Melnick J. L. Concentration of enteroviruses from estuarine water. Appl Environ Microbiol. 1977 May;33(5):1192–1196. doi: 10.1128/aem.33.5.1192-1196.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Farrah S. R., Goyal S. M., Wallis C., Melnick J. L. Concentration of enteroviruses from large volumes of tap water, treated sewage, and seawater. Appl Environ Microbiol. 1978 Mar;35(3):540–548. doi: 10.1128/aem.35.3.540-548.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., McLeod J. S. Effect of sediments on the survival of Escherichia coli in marine waters. Appl Environ Microbiol. 1976 Jul;32(1):114–120. doi: 10.1128/aem.32.1.114-120.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P., Melnick J. L. Occurrence and distribution of bacterial indicators and pathogens in canal communities along the Texas coast. Appl Environ Microbiol. 1977 Aug;34(2):139–149. doi: 10.1128/aem.34.2.139-149.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Melnick J. L., Wallis C. Virus isolations from sewage and from a stream receiving effluents of sewage treatment plants. Bull World Health Organ. 1970;42(2):291–296. [PMC free article] [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Effect of sodium bicarbonate concentration on plaque formation of virulent and attenuated polioviruses. J Immunol. 1958 Apr;80(4):282–293. [PubMed] [Google Scholar]
- Koff R. S., Grady G. F., Chalmers T. C., Mosley J. W., Swartz B. L. Viral hepatitis in a group of Boston hospitals. 3. Importance of exposure to shellfish in a nonepidemic period. N Engl J Med. 1967 Mar 30;276(13):703–710. doi: 10.1056/NEJM196703302761301. [DOI] [PubMed] [Google Scholar]
- Liu O. C., Seraichekas H. R., Murphy B. L. Viral pollution of shellfish. 1. Some basic facts of uptake. Proc Soc Exp Biol Med. 1966 Nov;123(2):481–487. doi: 10.3181/00379727-123-31520. [DOI] [PubMed] [Google Scholar]
- MASON J. O., McLEAN W. R. Infectious hepatitis traced to the consumption of raw oysters. An epidemiologic study. Am J Hyg. 1962 Jan;75:90–111. doi: 10.1093/oxfordjournals.aje.a120238. [DOI] [PubMed] [Google Scholar]
- METCALF T. G., STILES W. C. THE ACCUMULATION OF ENTERIC VIRUSES BY THE OYSTER, CRASSOSTREA VIRGINICA. J Infect Dis. 1965 Feb;115:68–76. doi: 10.1093/infdis/115.1.68. [DOI] [PubMed] [Google Scholar]
- Mackowiak P. A., Caraway C. T., Portnoy B. L. Oyster-associated hepatitis: lessons from the Louisiana experience. Am J Epidemiol. 1976 Feb;103(2):181–191. doi: 10.1093/oxfordjournals.aje.a112216. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Rennick V., Hampil B., Schmidt N. J., Ho H. H. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 42 enteroviruses. Bull World Health Organ. 1973;48(3):263–268. [PMC free article] [PubMed] [Google Scholar]
- Metcalf T. G., Stiles W. C. Enteroviruses within an estuarine environment. Am J Epidemiol. 1968 Nov;88(3):379–391. doi: 10.1093/oxfordjournals.aje.a120898. [DOI] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Wallis C., Melnick J. L. Development of a simple method for concentrating enteroviruses from oysters. Appl Microbiol. 1975 Jan;29(1):21–26. doi: 10.1128/am.29.1.21-26.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Concentration of viruses from sewage by adsorption on millipore membranes. Bull World Health Organ. 1967;36(2):219–225. [PMC free article] [PubMed] [Google Scholar]


