Abstract
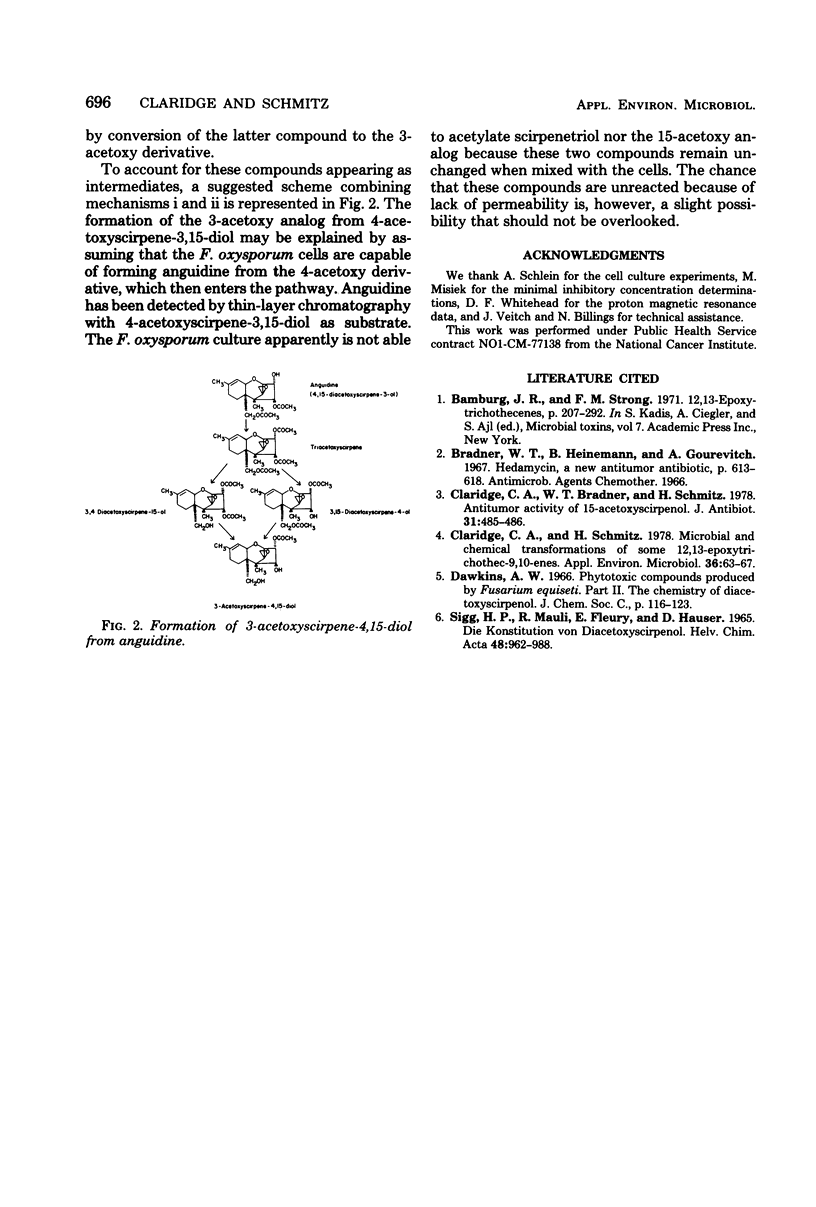
Growing cells of Fusarium oxysporum f.sp. vasinfectum (ATCC 7808) formed 3-acetoxyscirpene-4,15-diol from anguidine (4,15-diacetoxyscirpene-3-ol) by way of the intermediates triacetoxyscirpene, 3,4-diacetoxyscirpene-15-ol and 3,15-diacetoxyscirpene-4-ol. The new 3-acetoxy analog was found to be less active than anguidine and the other monoacetoxy derivatives when tested against a series of fungal strains and against HeLa cells in vitro.
Full text
PDF
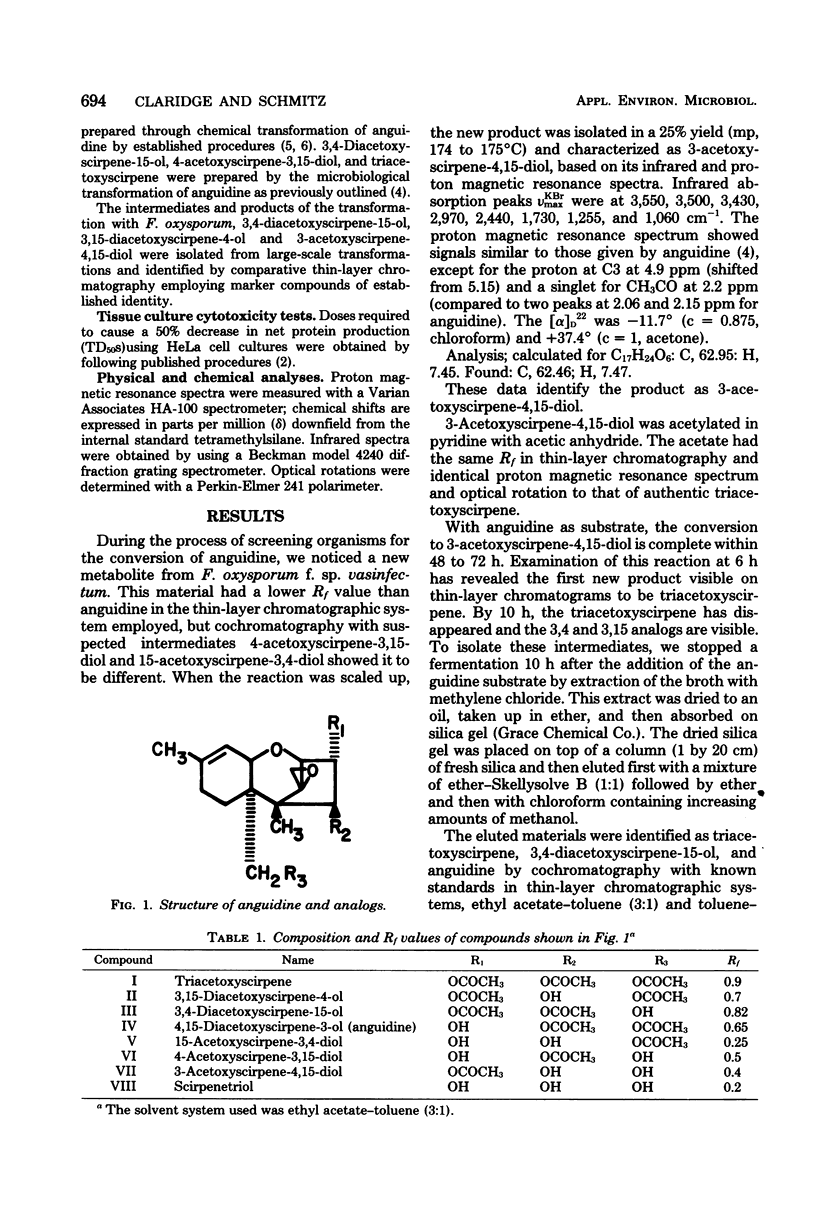

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claridge C. A., Bradner W. T., Schmitz H. Antitumor activity of 15-acetoxyscirpen-3,4-diol. J Antibiot (Tokyo) 1978 May;31(5):485–486. doi: 10.7164/antibiotics.31.485. [DOI] [PubMed] [Google Scholar]
- Claridge C. A., Schmitz H. Microbial and chemical transformations of some 12,13-epoxytrichothec-9,10-enes. Appl Environ Microbiol. 1978 Jul;36(1):63–67. doi: 10.1128/aem.36.1.63-67.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


