Abstract
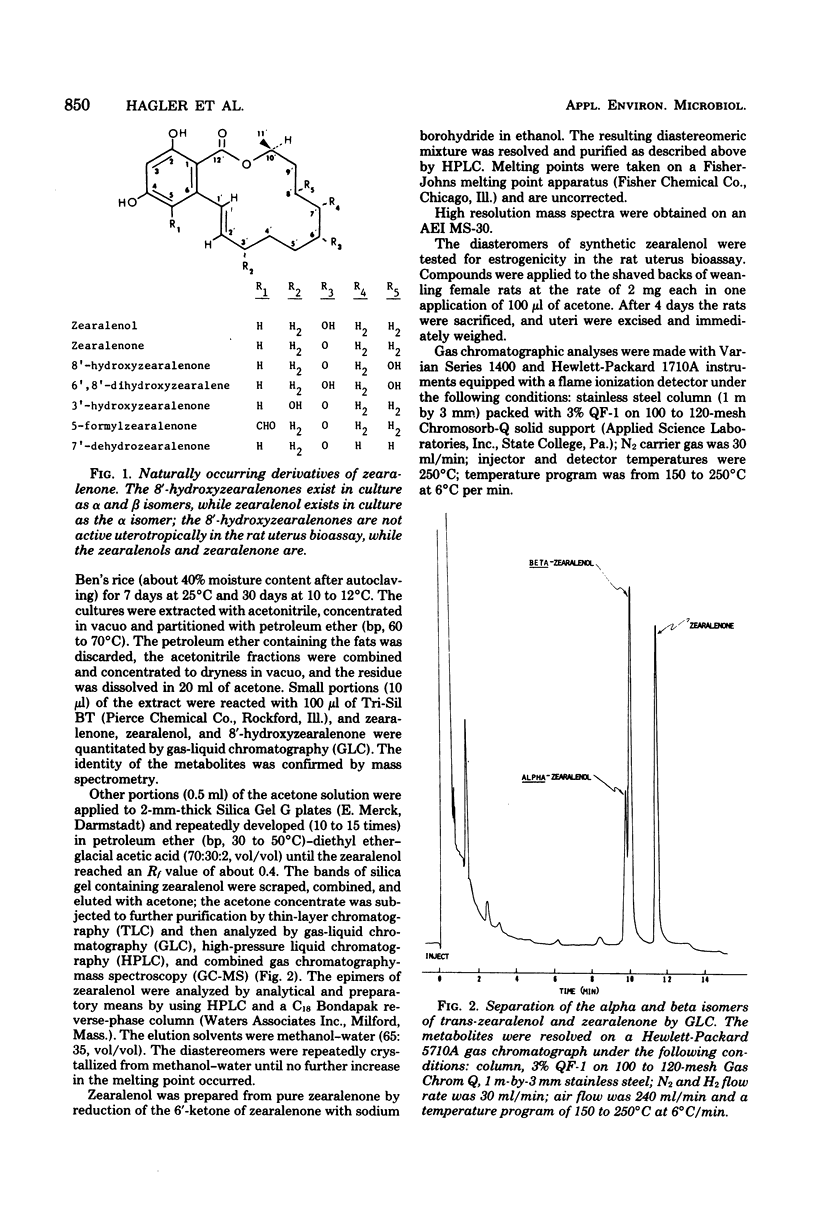
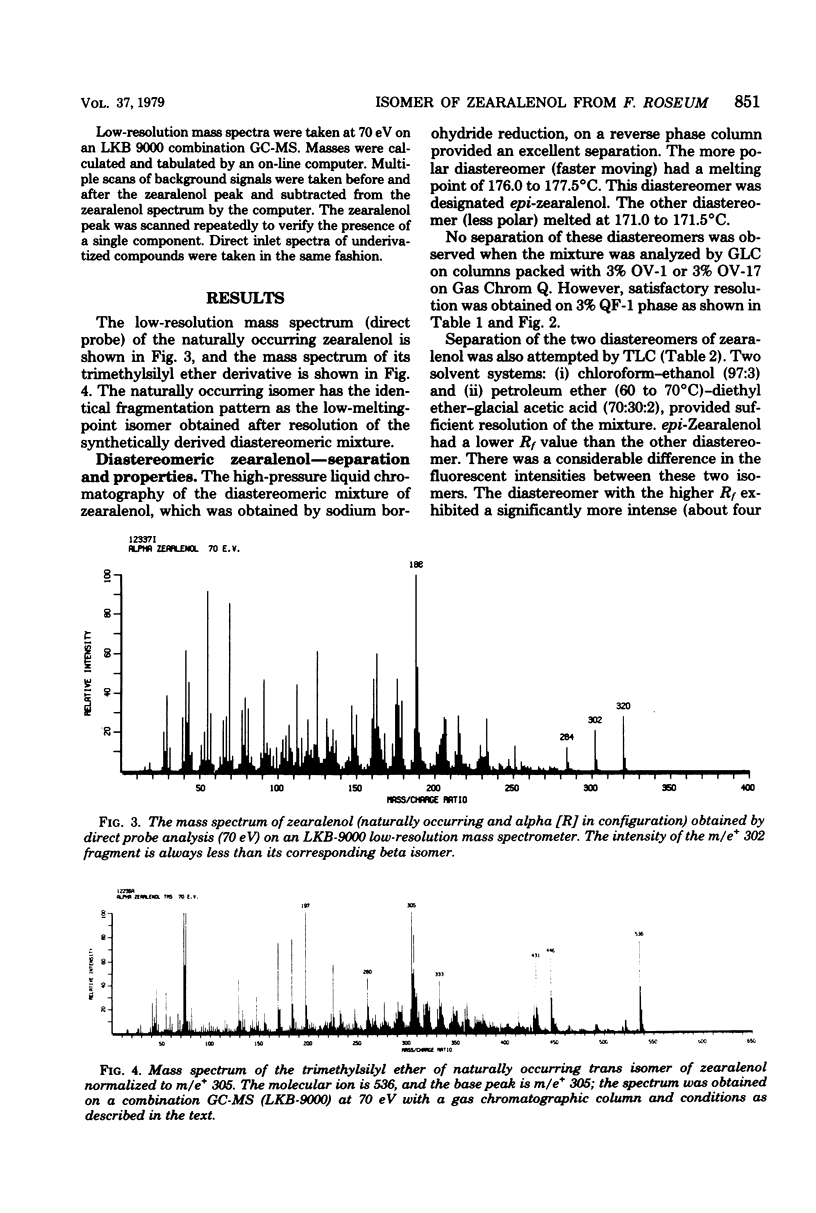
One diastereomer of trans-zearalenol [2,4-dihydroxy-6-(6,10-dihydroxy-trans-1-undecenyl)-benzoic acid-mu-lactone] was isolated from cultures of Fusarium roseum 'Gibbosum.' This strongly estrogenic metabolite was identified by analysis of its mass spectrum and its behavior in thin-layer, high-pressure liquid and gas-liquid chromatographic systems. The concentration of zearalenol in cultures was 563 mu g/g, or 7% of the 8,000-mu g/g zearalenone content, while the two diastereomers of 8'-hydroxyzearalenone each occurred at 3% of the zearalenone level. Of the two possible diastereomers of zearalenol, the one occurring in cultures was identical to the low-melting-point (171 degrees C) isomer (alpha) obtained by synthesis. In the rat uterus bioassay, the alpha zearalenol isomer was three times more estrogenic than zearalenone while the beta isomer was equal in activity in zearalenone. The two diastereomers of zearalenol can be distinguished from each other by the intensity of the m/e+ 302 fragment of the mass spectrum of the pure underivatized compound.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hidy P. H., Baldwin R. S., Greasham R. L., Keith C. L., McMullen J. R. Zearalenone and some derivatives: production and biological activities. Adv Appl Microbiol. 1977;22:59–82. doi: 10.1016/s0065-2164(08)70160-6. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Fenton S. W., Mirocha C. J., Davis G. Characterization of two isomers of 8'-hydroxyzearalenone and other derivatives of zearalenone. J Agric Food Chem. 1974 Nov-Dec;22(6):1015–1019. doi: 10.1021/jf60196a039. [DOI] [PubMed] [Google Scholar]
- Peters C. A. Photochemistry of zearalenone and its derivatives. J Med Chem. 1972 Aug;15(8):867–868. doi: 10.1021/jm00278a028. [DOI] [PubMed] [Google Scholar]
- Steele J. A., Mirocha C. J., Pathre S. V. Metabolism of zearalenone by Fusarium roseum Graminearum. J Agric Food Chem. 1976 Jan-Feb;24(1):89–97. doi: 10.1021/jf60203a001. [DOI] [PubMed] [Google Scholar]
- Stipanovic R. D., Schroeder H. W. Zearalenol and 8'-hydroxyzearalenone from Fusarium roseum. Mycopathologia. 1975 Dec 23;57(2):77–78. doi: 10.1007/BF01365707. [DOI] [PubMed] [Google Scholar]


