Abstract
Synthetic oligodeoxynucleotides containing unmethylated CpG sequences (CpG-ODNs) stimulate Toll-like receptor-9 (TLR-9), thereby activating innate immunity. Stimulatory CpG-ODNs have been shown to be valuable in modifying immune responses in allergy, infection and cancer. Recently, it has been reported that the stimulation of TLR-9 by endogenous DNA might contribute to the pathogenesis of autoimmune diseases. We here report the identification of a suppressive, guanosine-rich ODN (G-ODN) that inhibited the activation of TLR-9 by stimulatory CpG-ODNs. The G-ODN was suppressive in murine macrophages and dendritic cells as well as in human plasmacytoid dendritic cells in vitro. G-ODN blocked the secretion of tumour necrosis factor-α (TNF-α) and interleukin-12p40 and interfered with the up-regulation of major histocompatibility complex (MHC) class II and costimulatory molecules. G-ODN was inhibitory even at a molar ratio of 1 : 10 (G-ODN:CpG-ODN) and when administered up to 7 hr after stimulation with CpG. G-ODN specifically inhibited TLR-9 but not other TLRs. Inhibition was dependent on a string of five guanosines. G-ODN was also inhibitory in an in vivo model of CpG/galactosamin (GalN) lethal shock. G-ODN interfered with upstream TLR-9 signalling. However, by extensive analysis we can exclude that G-ODN acts at the stage of cellular uptake. G-ODN therefore represents a class of suppressive ODNs that could be of therapeutic use in situations with pathologic TLR-9 activation, as has been proposed for certain autoimmune diseases.
Keywords: CpG oligodeoxynucleotides, inhibition, innate immunity, suppressive oligodeoxynucleotides, Toll-like receptor-9
Introduction
Toll-like receptors mediate activation of the innate immune system by recognizing conserved microbial structures. Toll-like receptor-9 (TLR-9) has been shown to be stimulated by bacterial DNA, which, in contrast to vertebrate DNA, is characterized by the frequent occurrence of unmethylated CG dinucleotides.1,2 It is generally assumed that TLR activation helps in the defence against invading pathogens. However, an essential contribution of TLR-9 to combat microbes has only been reported for a limited number of pathogens, including a few bacteria,3,4 viruses5,6 and parasites.7–9 Initially it was thought that TLR-9 specifically recognizes foreign bacterial DNA, but not self-DNA. However, it is now understood that, under special conditions, vertebrate DNA is able to trigger TLR-9. DNA from dying eukaryotic cells was reported to activate immune responses dependent on TLR-9.10 Moreover, intracellular localization of TLR-9 seems to restrain the specificity of DNA recognition. Under physiological conditions, endosomal expression of TLR-9 is observed and this helps to prevent activation by self-DNA.11 Delivery of vertebrate DNA into the endosome, or expression of TLR-9 on the cell membrane, in turn resulted in immunostimulation.12,13
There are now several reports that assign TLR-9 an important role in autoimmune diseases via activation by self-DNA.14,15 It has been observed that B lymphocytes with rheumatoid factor specificity are triggered by chromatin/immunoglobulin G (IgG) complexes in a TLR-9-dependent manner.16,17 Chloroquine, an inhibitor of endosomal acidification, has been known for a number of years to have beneficial effects on rheumatoid arthritis and has been shown to inhibit TLR-9 signalling.18 Moreover, TLR-9 activation of plasmacytoid dendritic cells (DC), which results in the elevated production of type I interferons (IFNs), has been reported to contribute to systemic lupus erythematosus.13,19
TLR-9 activation can also be achieved by the application of short, synthetic oligodeoxynucleotides containing a CpG motif (CpG-ODNs).1 Extensive analysis during the last few years has identified three different classes of CpG-ODNs based upon their predominant activation of either B lymphocytes or plasmacytoid dendritic cells (pDC).20 Meanwhile, CpG-ODNs are in clinical trials for the treatment of cancer, allergy and infections.20 However, with the recent identification of a role of TLR-9 in autoimmune diseases, considerable interest for antagonists of CpG DNA or inhibitors of TLR-9 has evolved.
The first observations towards inhibition of TLR-9 came from Krieg et al., who observed that certain adenoviral sequences not only lacked immunostimulatory capacity but even inhibited CpG-ODN-mediated immune activation.21 Furthermore, methylated CG motifs were shown to be inhibitory for bacterial DNA stimulation22 and similar findings were observed for telomeric DNA repeats.23 It was also shown that poly guanosine-rich sequences interfered with CpG activation;22,24,25 however, this inhibitory effect was not specific for TLR-9. Thus, it became clear that inhibition of TLR-9 can also be mediated by synthetic ODNs. Over the last few years the group of Lenert has focused on the characterization of a specific group of inhibitory ODNs.26–29 They reported that short, up to 15-bp-long, ODNs with pyrimidine-rich triplets followed by a GGG sequence specifically inhibited TLR-9 signalling,30 and this was confirmed by others.24,31
However, the exact mode of inhibition has not yet been elucidated. Also, macrophages and DC have not been analysed in detail. Moreover, the confusing variety of DNA sequences and structures reported to inhibit TLR-9 signalling makes it difficult to compare the published studies. Therefore, it was recently proposed that inhibitory ODNs might be classified in four different groups that differ in terms of TLR-9 specificity, competition for cellular uptake of ODNs, TLR-9 binding and effectiveness.32 Group I inhibitory ODNs included short guanosine-rich sequences, which seem to show the most promise of specific TLR-9 inhibition. Accordingly, it would be favourable to evaluate each of the different groups of inhibitory ODNs fully under the criteria given above.
We describe here the identification of such a group I inhibitory ODN, which subsequently was analysed in detail in terms of inhibitory potency, kinetics, TLR specificity and mode of action in macrophages and DC. Moreover, this suppressive ODN was examined for its functionality in vivo.
Materials and methods
Reagents
ODNs were custom synthesized by MWG-Biotech AG (Ebersberg, Germany). We observed marked differences in the quality of synthesis of poly guanosine strings between different companies. Sequences are displayed in Table 1. RNA9.2 (AGCUUAACCUGUCCUUCAA)33 was custom synthesized by IBA (Göttingen, Germany). Highly purified lipopolysaccharide (LPS) was prepared from Salmonella minnesota (HL63, smooth form) and was a gift from U. Seydel (Research Center Borstel, Borstel, Germany). Pure lipoteichoic acid was from Staphylococcus aureus and was received from Thomas Hartung (European Centre for the Validation of Alternative Methods (ECVAM), Ispra, Italy). Poly [I:C] and imiquimod (R837) were purchased from Invivogen (San Diego, CA). Pam3CSK4 and macrophage-activating lipopeptide-2 (MALP2) were obtained from Alexis (Lausen, Switzerland). Transfection reagent N-[1-(2,3-dioleoyloxy)]-N,N,N-trimethylammonium propan methylsulfate (DOTAP) was from Roth (Karlsruhe, Germany). Phosphospecific antibodies to mitogen-activated protein (MAP) kinases were received from Cell Signaling Technology (Danvers, MA, USA), and the antibody against actin was from Abcam (Cambridge, UK).
Table 1.
Sequences of oligodeoxynucleotides
| Name | Sequence (5′−3′)1 | Length (bp) | CpG motif | Poly G motif |
|---|---|---|---|---|
| CpG-1668 | tccatgacgttcctgatgct2 | 20 | + | – |
| G-ODN | ctcctattgggggtttcctat | 21 | – | + |
| GC-ODN | tccatgagcttcctgatgct | 20 | – | – |
| CpG-2216 | ggGGGACGATCGTCgggggg | 20 | + | – |
| CpG-2006 | tcgtcgttttgtcgttttgtcgtt | 24 | + | – |
| G5 | ggggg | 5 | – | + |
| G*-ODN | ctcctattgtgtgtttcctat | 21 | – | Disrupted |
| CpG-G-ODN | tccatgacgttcctgatgctggggg | 25 | + | + |
| CpG-G*-ODN | tccatgacgttcctgatgctagaga | 25 | + | Disrupted |
Underlined are poly guanosine, inhibitory motifs; stimulatory CpG-sequences are displayed in bold. Phosphodiester (PO) bonds are in capital letters and phosphorothioate (PTO) bonds are in lower case.
CpG-1668 was also used in the phosphodiester form (PO-CpG).
Cells and culture conditions
RAW 264.7 cells, a murine macrophage cell line, were a kind gift from R. Schumann (Charité, Berlin, Germany). HEK293 cells were obtained from S. Bauer (Institute for Immunology, Marburg, Germany). The cells were cultured in RPMI 1640 (RAW264.7 cells) or in Dulbecco's modified Eagle's minimal essential medium (DMEM) (HEK293 cells) from Biochrom (Berlin, Germany) supplemented with 5 or 10% fetal calf serum (FCS), respectively, 50 µm 2-mercaptoethanol and antibiotics (penicillin G and streptomycin). HEK-TLR-9 cells were obtained by transfecting HEK293 cells with a murine TLR-9-YFP expression plasmid (obtained from T. Espevik, Institute of Cancer Research and Molecular Medicine, Trondheim, Norway) and selection for stable expression in 0·8 mg/ml of G418. Cells were cloned and analysed for CpG DNA responsiveness.
Bone marrow-derived dendritic cells (BMDC) were prepared from female, 4- to 10-week-old, BALB/c mice, as described by Inaba et al.,34 with minor modifications. Briefly, bone marrow cells were placed in 70-cm2 tissue-culture flasks in differentiation medium: RPMI 1640 supplemented with 10% FCS, 50 mm 2-mercaptoethanol, penicillin G (100 IU/ml), streptomycin sulfate (100 IU/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (200 U/ml). After 24 hr, non-adherent cells were collected, washed and 107 of these cells were seeded into 175-cm2 tissue-culture flasks in differentiation medium. On day 5, fresh differentiation medium was added, and on day 9 non-adherent, immature DC (> 90% CD11c+ B220–) were harvested. The culture supernatant of a GM-CSF-transfected cell line was also used as a source of GM-CSF.
Peripheral blood mononuclear cells (PBMCs) were isolated from the heparinized blood of healthy donors by standard Ficoll–Paque density-gradient centrifugation and washed three times in phosphate-buffered saline (PBS) supplemented with 5 mm EDTA. Local ethical committee approval was received and informed consent of donors was obtained.
Western blotting
Cells were lysed for 30 min on ice in 250 µl of lysis buffer (50 mm Tris-HCl, pH 7·4; 1% Igepal; 0·25% sodium deoxycholate; 150 mm NaCl; 1 mm EDTA; 1 mm phenylmethylsulfonylfluoride (PMSF); 1 µg/ml each of aprotinin, leupeptin and pepstatin; 1 mm Na3VO4; and 1 mm NaF). Lysates were cleared by centrifugation (4°, 10 min, 11 000 g). Equal amounts of lysates were fractionated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) (12% gel) and electrotransferred to polyvinylidene difluoride membranes. The membranes were blocked with Tris-buffered saline (TBS) (pH 7·8)/5% non-fat dry milk/0·05% Tween 20, and were blotted with the indicated antibodies according to the manufacturer's protocol. Horseradish peroxidase (HRP)-labelled antibodies were visualized by enhanced chemiluminescence (ECL) (Amersham, Freiburg, Germany).
Flow cytometry and uptake measurement
Flow cytometry staining was performed using antibodies from Becton Dickinson (Heidelberg, Germany). Cells were analysed on a FACS Canto (Becton Dickinson). For measuring the uptake of fluorescein isothiocyanate (FITC)-labelled ODNs, RAW264.7 cells were incubated with the indicated ODNs for 3 hr either at 4° or at 37°. Cells were washed three times in ice-cold PBS and analysed by flow cytometry. Dead cells were excluded by propidium iodide staining, and geometric mean fluorescence intensity (MFI) (FITC) was determined. Cellular uptake was quantified as follows: Uptake = MFI(37°) − MFI(4°). The mean values of at least five experiments are shown.
Cell stimulation and cytokine measurement
Cells – 2 × 105 (HEK-TLR-9, BMDC), 1·5 × 105 (RAW264.7) or 4 × 105 (PBMC) – were stimulated, as indicated in the respective experiments, in 96-well plates in duplicate overnight. RNA9.2 and phosphodiester (PO)-ODNs were delivered with DOTAP in a ratio of 1 : 4 according to the manufacturer's protocol. Cell-free supernatants were analysed for cytokine secretion by enzyme-linked immunosorbent assay (ELISA) (OptEIA; BD Pharmingen, Heidelberg, Germany). Shown are duplicates of one experiment out of three.
Reporter gene experiments
For reporter gene experiments, a luciferase reporter construct with a 6xNFκB responsive element was used. HEK293 cells were transfected in a 24-well format with 25 ng of TLR-9 or TLR-9N4C plasmid (obtained from Gregory M. Barton, Department of Molecular and Cell Biology, Berkeley, USA) and 25 ng of reporter gene using Lipofectamin 2000 (Invitrogen, Karlsruhe, Germany). Cells were stimulated 48 hr after transfection and, a further 6 hr later, luciferase activity was determined using the LucLite Kit from Perkin-Elmer (Wellesley, MA). The mean values of triplicates of one out of five experiments are shown.
Quantitative reverse transcription–polymerase chain reaction
Total RNA was isolated using Qiagen RNeasy (Qiagen, Valencia, CA) or the HighPure RNA kit (Roche, Mannheim, Germany), which included DNAse I digestion. A total of 1 µg of RNA was reverse transcribed into cDNA (MBI Fermentas, St Leon-Rot, Germany) using oligo(dT) primer. cDNA was diluted 1 : 4 and 2·5 µl was used as template in 25 µl of TaqMan-PCR mix, according to the manufacturer's protocol (reagents from AbGene, Hamburg, Germany; platform ABI Prism 7700; Applied Biosystems, Darmstadt, Germany). Quantifications were made using fluorogenic probes (FAM/TAMRA; Eurogentec, Seraing, Belgium). The specificity of the reverse transcription–polymerase chain reaction (RT–PCR) was controlled by no-template and no-reverse transcriptase controls. Quantitative PCR results were expressed relative to the housekeeping genes β-actin (murine cells) (1/2(Ct[target] − Ct[actin])) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (human cells). All primer sequences are available on request. The mean values of three experiments are shown.
In vivo models
To study lethal shock induction, mice were injected intraperitoneally (i.p.) with 20 mg of d-galactosamin (d-GalN; Roth) together with 2 µg of LPS (Escherichia coli 0127:B8; Sigma, Deisenhofen, Germany), 300 µg of E. coli DNA, 5 nmol CpG-ODN or with 10 µg of S. aureus Enterotoxin B (SEB; Toxin Technologies, Sarasota, FL). Inhibitory G-ODN was injected i.p., as indicated, at 10 nmol per mouse. Mice were monitored and lethality was recorded. Experiments were in accordance with local legislation.
Results
Poly guanosine-containing ODNs inhibit CpG-induced activation of macrophages and dendritic cells
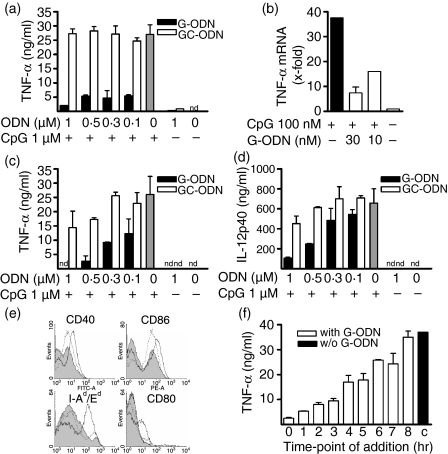
During our studies with various sequence-modified ODNs we identified an ODN that inhibited CpG-mediated activation of innate immune cells. This ODN was characterized by the presence of a 3′-string of five guanosines and contained no stimulatory CpG motif (named G-ODN). We observed that G-ODN, but not a control ODN in which the CpG dinucleotide was simply inverted to a non-stimulatory GpC sequence (GC-ODN), inhibited tumour necrosis factor-α (TNF-α) secretion of RAW264.7 macrophages induced by the well-characterized immunostimulatory CpG-ODN 1668 (Fig. 1a). G-ODN alone was not stimulatory. It effectively suppressed CpG-mediated TNF-α secretion, even when given in a molar ratio of 1 : 10. Closer examination revealed that G-ODN not only inhibited TNF-α protein secretion but also TNF-α mRNA induction by CpG-ODN (Fig. 1b). Again, the inhibitory effect was observed even with a 1 : 10 molar ratio (G-ODN:CpG-ODN), and 10 nm G-ODN was sufficient to diminish CpG-mediated TNF-α mRNA induction by 60%. We next analysed primary BMDC, which were co-incubated with stimulatory CpG-ODNs and either inhibitory G-ODN or neutral GC-ODN. CpG-mediated TNF-α secretion in DCs was inhibited by G-ODN in a dose-dependent manner (Fig. 1c). Again, a ratio of G-ODN:CpG-ODN of 1 : 10 still showed inhibition. GC-ODN showed slight inhibition only at high molar ratios. G-ODN, but not GC-ODN, also inhibited CpG-mediated interleukin (IL)-12p40 secretion in DC (Fig. 1d). However, IL-12p40 secretion was less effectively inhibited than secretion of TNF-α. CpG-ODN not only induces cytokine secretion in DCs but also up-regulates the expression of major histocompatibility complex (MHC) class II as well as costimulatory molecules. Incubation with G-ODN alone did not alter the expression of any examined surface marker, but significantly interfered with and inhibited the CpG-mediated up-regulation of CD40, CD80, CD86 and MHC class II (Fig. 1e).
Figure 1.
Guanosine-rich oligodeoxynucleotide (G-ODN) inhibits CpG-induced activation of macrophages and dendritic cells. (a) RAW264.7 cells were incubated overnight with 1 µm CpG-ODN in the presence of the indicated concentrations of G-ODN or GC-ODN, respectively. Tumor necrosis factor-α (TNF-α) was measured in the supernatant. (b) RAW264.7 cells were stimulated with 100 nm CpG-ODN in the presence of the indicated concentrations of G-ODN for 4 hr; mRNA was extracted and the relative expression of TNF-α was quantified by reverse transcription–polymerase chain reaction (RT-PCR). (c,d) Bone-marrow dendritic cells (BMDC) were stimulated overnight with 1 µm CpG-ODN in the presence of G-ODN or GC-ODN. TNF-α and interleukin (IL)-12p40 were determined in the supernatant. (e) BMDC were left untreated (solid grey) or were stimulated overnight with 1 µm CpG-ODN (thick line) and stained for surface expression of the indicated marker. Additionally, cells were stimulated with CpG-ODN in the presence of 0·3 µm G-ODN (dotted line). (f) RAW264.7 cells were stimulated with 1 µm CpG-ODN. At the indicated time, 0·3 µm G-ODN was added. Sixteen hours after stimulation, TNF-α was determined in the supernatant (mean plus standard deviation, one representative experiment out of at least three independent experiments). nd, not determined.
Delayed addition of G-ODN inhibits CpG-mediated TNF-α secretion
We next examined the inhibitory potency of G-ODN when given delayed to CpG-ODN-stimulated cells (Fig. 1f). We observed that although the co-administration of G-ODN and CpG-ODN was most effective at blocking TNF-α secretion in RAW264.7 macrophages, even delayed administration, up to 7 hr later, still resulted in partial inhibition.
Suppressive G-ODN specifically inhibits TLR-9
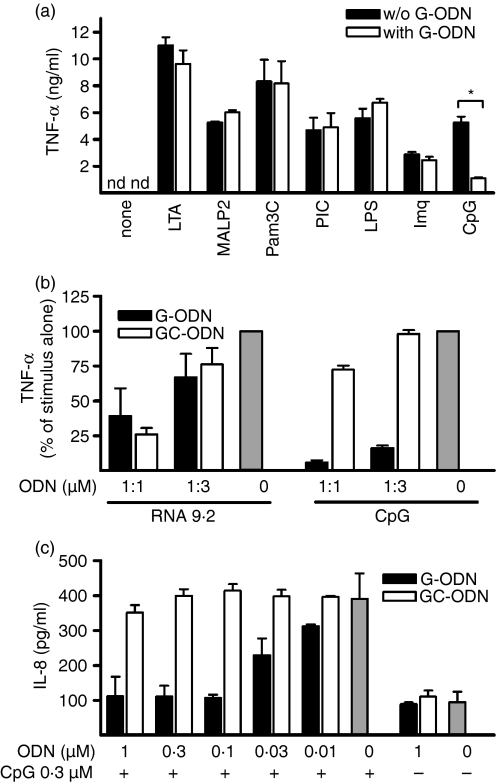
We next assessed whether the inhibitory effect of G-ODN was specific for TLR-9 as this has been matter of debate in the literature. We co-incubated macrophages with G-ODN and specific ligands for TLR-1 and -2 (Pam3CSK4), TLR-2 (lipoteichoic acid), TLR-2 and -6 (MALP2), TLR-3 (poly[I:C]), TLR-4 (LPS), TLR-7 (imiquimod) and TLR-9 (CpG) at suboptimal concentrations and measured TNF-α secretion (Fig. 2a). We observed that G-ODN specifically inhibited activation via TLR-9, but none of the other tested TLRs. As TLR-9 shares with TLR-7 the property of being triggered in endosomes but not at the cell surface, and of recognizing nucleic acids, we examined TLR-7 activation more closely. Therefore, TLR-7 stimulation was performed by the addition of stimulatory RNA, which was delivered using the transfection agent DOTAP. We observed that at high molar ratios, G-ODN as well as GC-ODN reduced RNA-mediated immunostimulation (Fig. 2b), possibly by interfering with endosomal delivery. Yet, in comparison, stimulation with CpG-ODN was inhibited specifically by G-ODN at a much lower molar ratio and not by GC-ODN. Thus, we conclude that G-ODN specifically inhibited TLR-9 but not TLR-7, as also indicated by the experiment with imiquimod (Fig. 2a). To test TLR-9 specificity with an additional method we used otherwise TLR-9-deficient HEK293 cells. These cells can be transfected with individual TLRs and then specifically produce IL-8. Expression of TLR-9 in this system was sufficient for G-ODN, but not for GC-ODN, to inhibit TLR-9 stimulation (Fig. 2c).
Figure 2.
Guanosine-rich oligodeoxynucleotide (G-ODN) specifically inhibits Toll-like receptor 9 (TLR-9). (a) RAW264.7 macrophages were stimulated overnight with lipoteichoic acid (LTA) (10 µg/ml), macrophage-activating lipopeptide-2 (MALP2) (500 ng/ml), Pam3CSK4 (Pam3C; 5 µg/ml), poly[I:C] (PIC; 10 µg/ml), lipopolysaccharide (LPS) (10 ng/ml), imiquimod (Imq; 5 µg/ml) or CpG-ODN (CpG; 0·3 µm) in the presence or absence of G-ODN (0·03 µm). Tumor necrosis factor-α (TNF-α) was measured in the supernatant. nd, not determined. (b) RAW264.7 cells were stimulated with CpG-ODN or RNA in the presence of G-ODN and GC-ODN. RNA was delivered to the cells by use of N-[1-(2,3-dioleoyloxy)]-N,N,N-trimethylammonium propan methylsulfate (DOTAP). TNF-α was measured in overnight supernatants. DOTAP alone did not induce TNF-α secretion (c) HEK293 cells stably transfected with murine TLR-9 were incubated overnight with the indicated ODN, and interleukin-8 (IL-8) levels in the supernatant were measured.
G-ODN is suppressive in vivo
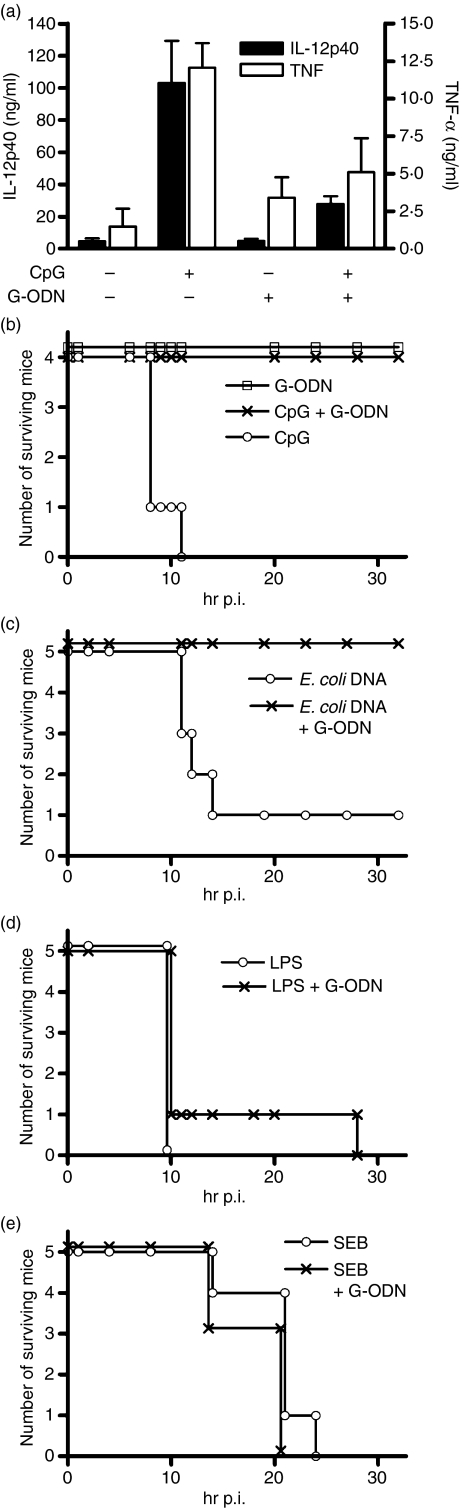
Having established that G-ODN is suppressive in macrophages and DC in vitro, we next analysed its effects in vivo. Similarly to the in vitro observations, the co-injection of CpG-ODN and G-ODN resulted in almost complete loss of serum TNF-α and IL-12p40 compared with injection of CpG-ODN alone (Fig. 3a). G-ODN displayed marginal induction of TNF-α, but not of IL-12p40 when given alone. Next, we analysed the efficacy of G-ODN in a model of cytokine-mediated lethal shock. Mice that were presensitized with GalN were injected either with agonists for TLR-4 (LPS) or for TLR-9 (CpG-ODN; E. coli DNA) or with SEB, which is a TLR-independent model (Fig. 3b–e). G-ODN completely inhibited lethality induced by CpG-ODN or by E. coli DNA. In contrast, G-ODN did not affect LPS- or superantigen-mediated lethality. This indicates that G-ODN is suppressive in vivo and specifically inhibits TLR-9-dependent cytokine responses.
Figure 3.
Guanosine-rich oligodeoxynucleotide (G-ODN) is effective in vivo and rescues mice from lethal Toll-like receptor 9 (TLR-9)-induced cytokine shock. (a) A 10-nmol sample of the indicated ODN was injected intraperitoneally (i.p.) into BALB/c mice. After 1·5 hr, tumor necrosis factor-α (TNF-α) and interleukin (IL)-12p40 levels were determined in serum (n = 3, mean + standard deviation). (b–e) Mice were injected i.p. with d-galactosamin (GalN) and with CpG-ODN (b), Escherichia coli DNA (c), lipopolysaccharide (LPS) (d) or Staphylococcus aureus enterotoxin B (SEB) (e) G-ODNs were injected concomitantly and lethality was recorded.
Suppressive G-ODN inhibits human plasmacytoid dendritic cells
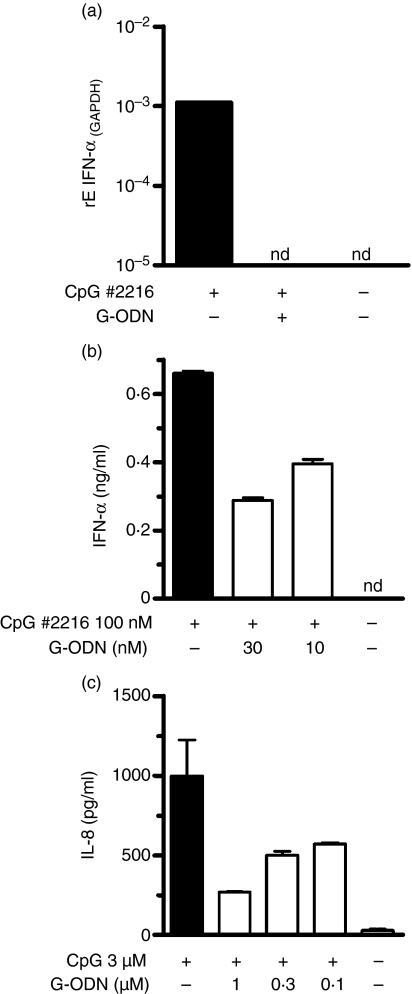
We next assessed whether the here-described suppressive G-ODN would also affect human cells. Therefore, human PBMCs were stimulated with A-type CpG-ODN 2216, which predominantly activates pDC. We measured the secretion of IFN-α as a specific readout for pDCs. G-ODN completely abolished CpG-induced mRNA of the measured IFN-α species (αΑ, α13, αD, α1B, α2, α1) at a given time-point (Fig. 4a). The findings were confirmed by an inhibition of IFN-α protein secretion to ≈ 50% (Fig. 4b) upon overnight stimulation. We also tested the inhibitory effect of G-ODN on HEK293 cells transfected with human TLR-9 and observed, at a ratio of 1 : 3, inhibition on CpG-induced IL-8 secretion of 70%. Thus, we conclude that suppressive G-ODNs are operative in both murine and human systems.
Figure 4.
Guanosine-rich oligodeoxynucleotide (G-ODN) inhibits human Toll-like receptor 9 (TLR-9) activation. (a) Human peripheral blood mononuclear cells (PBMCs) were incubated with the indicated ODN for 4 hr; mRNA was extracted and the expression of interferon-α (IFN-α) (as an indicator of plasmacytoid dendritic cell stimulation) was quantified by reverse transcription–polymerase chain reaction (RT–PCR). GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (b) PBMCs were stimulated overnight with 0·1 µm CpG 2216 in the presence of G-ODN, as indicated, and IFN-α was quantified by enzyme-linked immunosorbent assay (ELISA) in the supernatant. (c) HEK293 cells transfected with human TLR-9 were stimulated overnight with CpG 2006 in the presence or absence of G-ODN. Interleukin-8 (IL-8) was determined in cell-free supernatant. nd, not determined.
Sequence characteristics of suppressive G-ODN
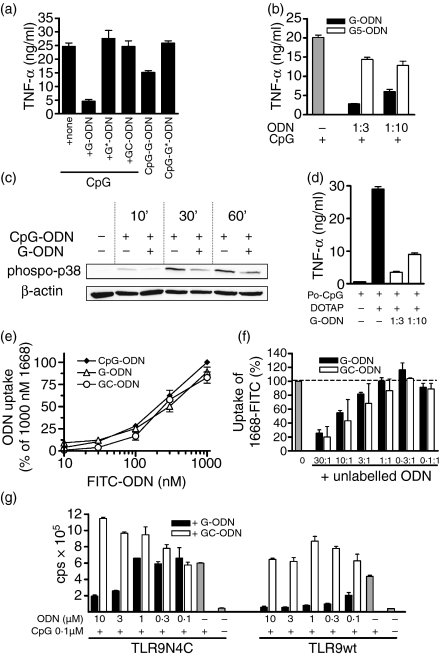
Next we analysed whether the string of five guanosines within G-ODN was essential for the observed suppressive effect (Fig. 5a). Disrupting the poly G string by interspersed thymidines (G*-ODN) abolished the inhibitory effect on CpG-induced TNF-α in macrophages and resulted in a non-stimulatory and non-inhibitory ODN as is GC-ODN. Strings of five guanosines alone (G5) were not sufficient to mediate the inhibitory effect (Fig. 5b), which indicates the requirement for longer sequences. G-ODN in our hands was only suppressive when used in a completely phosphorothioate (PTO) modified state (data not shown).
Figure 5.
Guanosine-rich oligodeoxynucleotide (G-ODN) effects are not mediated by inhibition of cellular uptake of CpG-ODN. (a) RAW 264.7 cells were stimulated overnight with 0·1 µm CpG, CpG-G-ODN or CpG-G*-ODN. In the case of CpG stimulation, cells were additionally co-incubated with 30 nm of the indicated inhibitory and control ODN. Tumor necrosis factor-α (TNF-α) was measured in the supernatant. (b) RAW264.7 cells were incubated overnight with 0·1 µm CpG-ODN alone or co-incubated with G-ODN and G-pentamere (G5), respectively. (c) Bone marrow-derived dendritic cells (BMDC) were stimulated with 1 µm CpG in the presence or absence of 0·3 µm G-ODN for the indicated time. Cell lysates were tested for phosphorylation of p38-mitogen-activated protein (MAP) kinase as well as expression of actin by western blotting. (d) RAW264.7 cells were stimulated overnight with 1 µm phosphodiester (PO)-CpG delivered with DOTAP. Cells were co-incubated, as indicated, with G-ODN. (e) RAW264.7 cells were incubated with the indicated amounts of fluorescein isothiocyanate (FITC)-labelled ODN for 3 hr and cellular uptake was quantified as described in the Materials and methods. Uptake of 1 µm CpG-ODN was set as 100%. (f) RAW264.7 cells were incubated with 1 µm FITC-labelled CpG-ODN in the presence or absence of unlabelled G-ODN and GC-ODN, respectively. Ratios of FITC-CpG-ODN and unlabelled ODN are indicated. Uptake was quantified by fluorescence-activated cell sorter (FACS) analysis and normalized to the uptake of 1 µm FITC-labelled CpG-ODN. (g) HEK293 cells were transiently transfected with murine Toll-like receptor 9 (TLR-9) or TLR-9N4C together with a nuclear factor-κB (NF-κB)-dependent luciferase reporter plasmid. Cells were stimulated for 6 hr with 0·1 µm CpG-ODN 1668 in the presence or absence of G-ODN or GC-ODN, respectively. Stimulation was determined by measuring luciferase activity. cps, counts per second.
G-ODN inhibits proximal CpG-ODN signalling
We next wanted to analyse the mode of inhibition of G-ODN. Initially we confirmed that G-ODN inhibited CpG-induced signalling events proximal of transcription induction. We observed that CpG-mediated phosphorylation of p38 MAP kinase was markedly inhibited by G-ODN (Fig. 5c). Co-incubation with CpG-ODN and G-ODN had shown the inhibitory effect of poly guanosine runs when delivered in trans. We next combined a stimulatory CpG-motif with a 3′-poly G run (named CpG-G-ODN) to test for inhibition in cis (Fig. 5a). This ODN showed a reduced stimulatory capacity when compared with the control ODN with a disrupted poly G motif (named CpG-G*-ODN). However, the inhibitory effect in cis was weaker compared with the addition in trans.
G-ODN-mediated suppression is not caused by interference with cellular delivery of CpG-ODN
We and others have previously reported that poly guanosine motifs enhance the cellular uptake of ODNs, which is a prerequisite for immunostimulation.35,36 However, it has to be stressed that this was only observed for PO ODN; PTO-modified ODN was taken up equally irrespective of the presence of poly G motifs. We next intended to analyse the role of cellular uptake in G-ODN inhibition. We first used delivery of PO-CpG-ODN by means of the transfection agent DOTAP, which bypasses the need for receptor-mediated uptake.12 Co-incubation of PO-CpG-ODN/DOTAP with G-ODN (PTO) showed a dramatic inhibition of TNF-α secretion, even when delivered in a 1 : 10 ratio (Fig. 5d). This indicates that G-ODN can inhibit CpG-ODN, even when cellular delivery occurs via a different route. Moreover, this confirms that G-ODN suppresses both PO- and PTO-modified CpG-ODN.
To determine in greater detail whether G-ODN would act on endosomal delivery of CpG-ODN, we directly compared the cellular uptake of FITC-labelled ODNs. We observed that CpG-, GC- and G-ODNs (all completely PTO modified) were taken up to a similar extent in macrophages (Fig. 5e). Moreover, neither unmarked GC-ODN nor unmarked G-ODN inhibited the cellular uptake of FITC-labelled CpG-ODN at low molar ratios (up to 1 : 1) (Fig. 5f). However, when given at 10 : 1 or higher excess, both ODNs inhibited the uptake of reporter FITC-CpG-ODN. This indicates that a so far unknown, but sequence-unspecific and saturable, ODN receptor mediates cellular uptake. However, this sharply contrasts the exclusive inhibitory effects of G-ODN (compare Fig. 1a), which occurred at much lower molar ratios (1 : 10 instead of excess). Therefore, we conclude that interference with cellular uptake is not responsible for the suppressive effects of G-ODN.
To strengthen this point further, we made use of a recently described TLR-9 construct that is composed of an extracellular TLR-9 domain coupled to an intracellular TLR-4 domain. This construct is expressed on the cellular surface and no longer in endosomal compartments.11 Having established that inhibition by G-ODN is operative in TLR-9-transfected HEK293 cells (compare Fig. 2c), we now analysed cells expressing either conventional TLR-9 (which requires endosomal uptake of CpG-ODN) or cell-surface TLR-9 (TLR-9N4C). HEK293 cells transfected with TLR-9N4C were highly responsive to PO-CpG-ODN in concentrations that, because of poor uptake of PO-ODN, led to only marginal activation of endosomal wild-type TLR-9 (data not shown). This confirms the extracellular localization of TLR-9N4C. G-ODN, but not GC-ODN, inhibited CpG-mediated induction of reporter gene activity in cells expressing the surface construct, TLR-9N4C (Fig. 5g). However, in comparison to the endosomal-expressed construct, this inhibition required higher concentrations of G-ODN. Yet, it has to be stressed that at these concentrations the inhibitory effect again was specific for G-ODN and was not observed for GC-ODN. These findings further support our conclusion that G-ODN do not operate at the level of cellular uptake.
Discussion
The activation of TLR-9 with synthetic CpG-ODNs has attracted considerable interest and clinical trials are now underway to evaluate the use of CpG-ODNs in the treatment of cancer, allergy and infections.20 These trials are based on the idea of strengthening activation of the innate immune systems and thereby modifying adaptive immune responses. However, with recent reports indicating that TLR-9 might also play a role in the pathogenesis of autoimmune diseases,13,16 the search for specific inhibitors of TLR-9 has been reinforced. Paralleling the approach of stimulating TLR-9 with synthetic CpG-ODNs, it was found that inhibitory or suppressive ODN exist, which not only fail to activate TLR-9 but that are even able to inhibit CpG-mediated activation.25,27,31
We here describe and characterize such a suppressive ODN, which would be classified, according to a recent proposal, as a group I inhibitory ODN.32 This suppressive ODN was inhibitory in murine macrophages and DC as well as in human pDCs. It showed considerable effectiveness and was able to inhibit at low molar ratios of G-ODN:CpG-ODN down to 1 : 30, thus being superior to formerly identified inhibitory ODN.31 Moreover, the here described suppressive ODN inhibited an already ongoing immune response, even when given up to 7 hr after CpG stimulation. This observation is not only of interest for possible therapeutic applications of G-ODN but also indicates that CpG-TLR-9 signalling is a relatively long ongoing process and does not follow a hit-and-run mechanism.
It is mainly the work of the groups of Lenert and Klinman that had already communicated the existence of inhibitory ODN.30,31 Lenart et al. reported the identification of a consensus ‘inhibitory’ motif.29 This turned out to be CCTN3−5GGG and, indeed, our inhibitory G-ODN sequence (-CCTATTGGGGG-) partially fits this sequence motif. However, of special importance is our observation that interruption of the string of five guanosine bases abrogates the inhibitory property (Fig. 5a). Thus, poly guanosine runs are a necessary (Fig. 5a), but not sufficient (Fig. 5b), structural requirement for inhibitory ODNs.25,27 G-tetrads have been reported to form higher-order intermolecular structures.37,38 However, disruption of these structures by deaza-modifications did not affect the inhibition of a strongly inhibitory ODN in mouse B cells.27
Inhibition at low molar ratios, need for a poly G motif and block at a proximal site in TLR-9 signalling (Figs 1a and 5a,c)26,28 raised the question of whether G-ODN interfere with the cellular uptake of CpG-ODN. Cellular uptake is a prerequisite for signalling of TLRs 7–9 because these receptors are not expressed on the cell surface but in intracellular membrane-bound vesicles.39–41 Our extensive analysis now clearly excludes specific interference of G-ODN with CpG-ODN uptake because we observed that inhibition of CpG signalling not only could be achieved by delivering G-ODN in trans but also by including poly G motifs in cis (CpG-G-ODN, Fig. 5a). The presence of inhibitory sequences in cis has also been suggested to explain the lack of stimulation of vertebrate genomic DNA.23,42 Moreover, bypassing receptor-mediated ODN uptake by delivery with the transfection agent DOTAP did not rescue from G-ODN inhibition (Fig. 5b). Also, cellular uptake of FITC-labelled G-ODN was almost identical to that of CpG-ODN (Fig. 5e) and G-ODN did not inhibit uptake of CpG-ODN in a competitive approach (Fig. 5f). Finally, we made use of a chimeric TLR-9 construct that has been reported to be expressed on the cell surface.11 We thereby circumvented the need of cellular uptake. Our observations of specific inhibition of surface-TLR-9 by G-ODN, but not by neutral GC-ODN, confirm that cellular uptake is not what is affected by suppressive ODN.
The results hint towards a direct interference at the receptor. However, it cannot be excluded that G-ODN directly interferes with CpG-ODN or affects binding to an intermediate adaptor protein. Our results fit to the observations of a disrupted TLR-9/CpG-ODN colocalization by inhibitory ODN.24 Inhibition of surface-expressed TLR-9 became visible only at higher molar ratios. This could be because endosomal delivery might elevate the local concentration of G-ODN relative to the extracellular concentration. Additionally, it was shown that CpG-ODN binding to TLR-9 was greater at lower pH, and thus the pH optima for G-ODN interaction may not be achieved at the surface.43 A recent report in which it was shown that CpG-DNA allosterically activates TLR-9, however, now shows that binding of inhibitory G-rich ODNs to TLR-9 is comparable to that of CpG-ODN.44 This indicates that inhibitory ODNs may act at still another, as-yet unknown, step in TLR-9 activation. Affecting vesicle trafficking is one possibility; however, our results using the surface-expressed TLR-9 do not support such a mechanism.
We also addressed the controversial issue of TLR specificity of inhibitory ODNs because it had been reported that ODNs with long poly G runs,25 or poly T ODNs,45 do not act in a mere TLR-9-specific manner. Our results clearly indicate that none of the other tested TLRs was inhibited. Especially, we show that TLR-7, which shares with TLR-9 its expression in endosomes, is not affected (Fig. 2a,b); thus, suppressive ODNs of the here-described class are TLR-9 specific. This is also confirmed by our in vivo experiments (Fig. 3). G-ODN completely inhibited CpG-ODN as well as bacterial DNA-mediated lethality in a shock model and this went along with inhibited TNF-α and IL-12p40 secretion. By contrast, no effects were observed for LPS- or SEB-induced lethality. These observations indicate that G-ODN can be used to block overshooting in vivo activity of TLR-9. Our findings of specific inhibition of TLR-9, but not of TLR-4, are in contrast to a publication from the Klinman group who reported inhibition of endotoxin shock by a different inhibitory ODN.46 Our results neither in vitro nor in vivo showed effects apart from inhibiting TLR-9. It might be that differences in the sequence of the ODNs used account for the contrasting findings; yet in terms of possible therapeutic application, a high specificity should be favourable.
This raises the question of possible applications of suppressive ODNs. As outlined above, stimulation of TLR-9 by endogenous DNA seems to play a role in various autoimmune diseases. Thus, suppressive ODN could be of use to block endogenous TLR-9 activation. This inhibition would be highly specific, and the lack of suppression of other TLRs would avoid the danger of increasing infection rates as a result of immunosuppression. Indeed, inhibitory ODNs have now been shown to block activation of lupus B cells by CpG-DNA.47 Moreover, repeated treatment of lupus-prone NZB/NZW mice with suppressive ODNs resulted in clinical improvement and delayed onset of glomerulonephritis.48 Furthermore, it was shown that suppressive ODNs had a beneficial effect in a model of CpG-induced arthritis,49 and it was reported subsequently that even in a collagen-induced arthritis model, treament with inhibitory ODNs resulted in improved clinical outcome.50 The results presented here, which use G-ODN in shock models, also indicate the usefulness of inhibitory G-ODN in sepsis settings with overshooting activation of innate immunity.
Acknowledgments
This project was supported by the Deutsche Forschungsgemeinschaft (DFG He1452/3 to K.H., DFG Da592/3 to A.D) and Landesstiftung Baden-Württemberg (P-LS-RNS/25). We thank Silvia Bendigs for her excellent technical support.
Abbreviations
- BMDC
bone marrow-derived dendritic cell
- DC
dendritic cell
- DOTAP
N-[1-(2,3-dioleoyloxy)]-N,N,N-trimethylammonium propan methylsulfate
- DMEM
Dulbecco's modified Eagle's minimal essential medium
- ECL
enhanced chemiluminescence
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- G-ODN
guanosine-rich ODN
- GalN
galactosamin
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HRP
horseradish peroxidase
- IFN-α
interferon-α
- IgG
immunoglobulin
- IL
interleukin
- i.p.
intraperitoneal
- MAP kinase
mitogen-activated protein kinase
- MHC
major histocompatibility complex
- MFI
mean fluorescence intensity
- ODN
oligodeoxynucleotide
- LPS
lipopolysaccharide
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- pDC
plasmacytoid dendritic cell
- PO
phosphodiester
- PTO
phosphorothioate
- RT–PCR
reverse transcription–polymerase chain reaction
- SEB
Staphylococcal enterotoxin B
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- TBS
Tris-buffered saline
- TNF-α
tumour necrosis factor-α
- TLR
Toll-like receptor
References
- 1.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 2.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 3.Kalis C, Gumenscheimer M, Freudenberg N, et al. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J Immunol. 2005;174:4295–300. doi: 10.4049/jimmunol.174.7.4295. [DOI] [PubMed] [Google Scholar]
- 4.Albiger B, Dahlberg S, Sandgren A, et al. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2006;9:633–44. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 5.Tabeta K, Georgel P, Janssen E, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–21. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–20. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minns LA, Menard LC, Foureau DM, et al. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–97. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 8.Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. Cutting Edge. TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–9. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 9.Pichyangkul S, Yongvanitchit K, Kum-arb U, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol. 2004;172:4926–33. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 10.Ishii KJ, Suzuki K, Coban C, et al. Genomic DNA released by dying cells induces the maturation of APCs. J Immunol. 2001;167:2602–7. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 11.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2005;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda K, Yu P, Kirschning CJ, et al. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J Immunol. 2005;174:6129–36. doi: 10.4049/jimmunol.174.10.6129. [DOI] [PubMed] [Google Scholar]
- 13.Barrat FJ, Meeker T, Gregorio J, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–9. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa HT, Williams LP, Segal BM. Activation of APCs through CD40 or Toll-like receptor 9 overcomes tolerance and precipitates autoimmune disease. J Immunol. 2002;169:2781–7. doi: 10.4049/jimmunol.169.5.2781. [DOI] [PubMed] [Google Scholar]
- 16.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 17.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Häcker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–40. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci USA. 2005;102:9258–63. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 21.Krieg AM, Wu T, Weeranta R, et al. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc Natl Acad Sci USA. 1998;95:12631–6. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pisetsky DS, Reich CF. Inhibition of murine macrophage IL-12 production by natural and synthetic DNA. Clin Immunol. 2000;96:198–204. doi: 10.1006/clim.2000.4897. [DOI] [PubMed] [Google Scholar]
- 23.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 24.Klinman DM, Zeuner R, Yamada H, Gursel M, Currie D, Gursel I. Regulation of CpG-induced immune activation by suppressive oligodeoxynucleotides. Ann NY Acad Sci. 2003;1002:112–23. doi: 10.1196/annals.1281.023. [DOI] [PubMed] [Google Scholar]
- 25.Halpern MD, Pisetsky DS. In vitro inhibition of murine IFN gamma production by phosphorothioate deoxyguanosine oligomers. Immunopharmacology. 1995;29:47–52. doi: 10.1016/0162-3109(95)00043-s. [DOI] [PubMed] [Google Scholar]
- 26.Lenert P, Stunz L, Yi AK, Krieg AM, Ashman RF. CpG stimulation of primary mouse B cells is blocked by inhibitory oligodeoxyribonucleotides at a site proximal to NF-kappaB activation. Antisense Nucleic Acids Drug Dev. 2001;11:247–56. doi: 10.1089/108729001317022241. [DOI] [PubMed] [Google Scholar]
- 27.Stunz LL, Lenert P, Peckham D, et al. Inhibitory oligonucleotides specifically block effects of stimulatory CpG oligonucleotides in B cells. Eur J Immunol. 2002;32:1212–22. doi: 10.1002/1521-4141(200205)32:5<1212::AID-IMMU1212>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Lenert P, Yi AK, Krieg AM, Stunz LL, Ashman RF. Inhibitory oligonucleotides block the induction of AP-1 transcription factor by stimulatory CpG oligonucleotides in B cells. Antisense Nucleic Acids Drug Dev. 2003;13:143–50. doi: 10.1089/108729003768247600. [DOI] [PubMed] [Google Scholar]
- 29.Lenert P, Rasmussen W, Ashman RF, Ballas ZK. Structural characterization of the inhibitory DNA motif for the type A (D)-CpG-induced cytokine secretion and NK-cell lytic activity in mouse spleen cells. DNA Cell Biol. 2003;22:621–31. doi: 10.1089/104454903770238094. [DOI] [PubMed] [Google Scholar]
- 30.Lenert P. Inhibitory oligodeoxynucleotides – therapeutic promise for systemic autoimmune diseases? Clin Exp Immunol. 2005;140:1–10. doi: 10.1111/j.1365-2249.2004.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada H, Gursel I, Takeshita F, et al. Effect of suppressive DNA on CpG-induced immune activation. J Immunol. 2002;169:5590–4. doi: 10.4049/jimmunol.169.10.5590. [DOI] [PubMed] [Google Scholar]
- 32.Trieu A, Roberts TL, Dunn JA, Sweet MJ, Stacey KJ. DNA motifs suppressing TLR9 responses. Crit Rev Immunol. 2006;26:527–44. doi: 10.1615/critrevimmunol.v26.i6.50. [DOI] [PubMed] [Google Scholar]
- 33.Hornung V, Guenthner-Biller M, Bourquin C, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–70. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 34.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SW, Song MK, Baek KH, et al. Effects of a hexameric deoxyriboguanosine run conjugation into CpG oligodeoxynucleotides on their immunostimulatory potentials. J Immunol. 2000;165:3631–9. doi: 10.4049/jimmunol.165.7.3631. [DOI] [PubMed] [Google Scholar]
- 36.Dalpke AH, Zimmermann S, Albrecht I, Heeg K. Phosphodiester CpG oligonucleotides as adjuvants. Poly-guanosine runs enhance cellular uptake and improve immunostimulative activity of phosphodiester CpG oligonucleotides in vitro and in vivo. Immunology. 2002;106:102–12. doi: 10.1046/j.1365-2567.2002.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benimetskaya L, Berton M, Kolbanovsky A, Benimetsky S, Stein CA. Formation of a G-tetrad and higher order structures correlates with biological activity of the RelA (NF-kappaB p65) ‘antisense’ oligodeoxynucleotide. Nucleic Acids Res. 1997;25:2648–56. doi: 10.1093/nar/25.13.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laughlan G, Murchie AI, Norman DG, Moore MH, Moody PC, Lilley DM, Luisi B. The high-resolution crystal structure of a parallel-stranded guanine tetraplex. Science. 1994;265:520–4. doi: 10.1126/science.8036494. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–68. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 40.Nishiya T, Kajita E, Miwa S, DeFranco AL. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J Biol Chem. 2005;280:37107–17. doi: 10.1074/jbc.M504951200. [DOI] [PubMed] [Google Scholar]
- 41.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 42.Stacey KJ, Young GR, Clark F, et al. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J Immunol. 2003;170:3614–20. doi: 10.4049/jimmunol.170.7.3614. [DOI] [PubMed] [Google Scholar]
- 43.Rutz M, Metzger J, Gellert T, Luppa P, Lipford GB, Wagner H, Bauer S. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur J Immunol. 2004;34:2541–50. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- 44.Latz E, Verma A, Visintin A, et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–9. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 45.Gorden KKB, Qiu X, Battiste JJL, Wightman PPD, Vasilakos JP, Alkan SS. Oligodeoxynucleotides differentially modulate activation of TLR7 and TLR8 by imidazoquinolines. J Immunol. 2006;177:8164–70. doi: 10.4049/jimmunol.177.11.8164. [DOI] [PubMed] [Google Scholar]
- 46.Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579–83. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- 47.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 48.Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB × NZW mice. Arthritis Rheum. 2005;52:651–8. doi: 10.1002/art.20810. [DOI] [PubMed] [Google Scholar]
- 49.Zeuner RA, Ishii KJ, Lizak MJ, Gursel I, Yamada H, Klinman DM, Verthelyi D. Reduction of CpG-induced arthritis by suppressive oligodeoxynucleotides. Arthritis Rheum. 2002;46:2219–24. doi: 10.1002/art.10423. [DOI] [PubMed] [Google Scholar]
- 50.Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligonucleotides protect against collagen-induced arthritis in mice. Arthritis Rheum. 2004;50:1686–9. doi: 10.1002/art.20263. [DOI] [PubMed] [Google Scholar]