Abstract
Mutations in nucleotide-binding oligomerization domain-2 (NOD2), leading to defective recognition of bacterial peptidoglycans, are associated with Crohn’s disease. The underlying mechanism that results in increased inflammation in the guts of the patients bearing NOD2 mutations is still unclear. We hypothesized that NOD2 engagement leads to cross-tolerance to stimulation of Toll-like receptors (TLR), and we investigated whether patients with Crohn’s disease who bear NOD2 mutations display a disturbed NOD2/TLR cross-tolerance. Peripheral blood mononuclear cells preincubated with NOD2 ligands were specifically down-regulated for the production of tumour necrosis factor-α (TNF-α) induced by the TLR4 ligand lipopolysaccharide, as well as by intestinal microorganisms, whereas the production of anti-inflammatory cytokines was not modulated. While in cells isolated from patients with Crohn’s disease with the wild-type NOD2 allele, the NOD2 engagement led to a similar cross-tolerance to TLR4-dependent stimulation of TNF-α, the cross-tolerance between NOD2 and TLR4 was absent in the cells of five patients homozygous for the 3020insC NOD2 mutation, leading to uninhibited release of TNF-α by TLR4 ligands and intestinal bacteria. In conclusion, we propose the absence of NOD2/TLR4 cross-tolerance as a central mechanism for the increased susceptibility to Crohn’s disease in individuals with NOD2 mutations.
Keywords: Crohn’s disease, NOD2, Toll-like receptors, tumour necrosis factor
Introduction
The IBD1 locus on chromosome 16 has been identified as a susceptibility locus for Crohn’s disease,1 and subsequently, nucleotide-binding oligomerization domain-2 (NOD2) has been identified as the candidate gene within the IBD1 locus.2–4 NOD2 is a member of the NACHT-LRR receptor (NLR) protein family, which is known to be involved in recognition of microbial structures, and is expressed intracellularly in antigen-presenting cells.5 Subsequent studies have identified NOD2 as the intracellular receptor for the muramyl dipeptide (MDP) component of peptidoglycan.6,7 However, the pathophysiological mechanisms responsible for the increased susceptibility to Crohn’s disease in patients with NOD2 mutations are unclear. Crohn’s disease patients who are homozygous for the 3020insC mutation of the NOD2 gene were found to have a defective release of cytokines after stimulation with peptidoglycan and its MDP components.8,9 How this defect paradoxically translates into increased local inflammation in the intestine has not been clarified. Several potential mechanisms have been proposed, including defective release of anti-inflammatory cytokines,8 or defective production of defensins, leading to bacterial overgrowth.10,11 Whereas a gain-of-function effect of the mutation on interleukin-1β (IL-1β) release was demonstrated in a mouse model,12 the latter mechanism was refuted in human studies.13 None of these mechanisms satisfactorily explains the increased inflammatory reaction found in the intestines of patients with Crohn’s disease.
In addition to its role as receptor for peptidoglycan, NOD2 also modulates signalling induced by Toll-like receptors (TLRs). Several studies have suggested that concomitant stimulation of NOD2 and TLR synergistically induces cytokines, although controversy exists around this issue.9,14 Such synergism between different receptors is a known amplification mechanism aimed at enforcing innate immunity. In addition, tolerance may be induced by repeated or chronic exposure to a stimulus. Such tolerance has been extensively described for bacterial lipopolysaccharide (LPS, endotoxin), and cross-tolerance to LPS and other stimuli at the level of TLR2 and TLR4 has been established.15,16 It is not known whether stimulation of NOD2 is able to induce cross-tolerance to TLR signalling. If this were the case, the chronic stimulation of NOD2 by intestinal peptidoglycans could tolerize for subsequent bacterial stimulation of TLRs, resulting in down-modulation of the proinflammatory cytokine response. Such a mechanism may contribute to maintaining intestinal homeostasis in the presence of a large quantity of bacterial stimuli.
Here we describe five patients who are homozygous for the 3020insC NOD2 mutation, and we demonstrate that NOD2 engagement leads to cross-tolerance to TLR4-dependent production of tumour necrosis factor-α (TNF-α), the central cytokine mediating inflammation in Crohn’s disease. The cross-tolerance between NOD2 and TLR4 was absent in the patients with the NOD2 mutation, and this leads to uninhibited release of TNF-α by intestinal bacteria and other TLR4 ligands. The tolerizing effect of NOD2 is specific for TLR4 stimulation; stimulation by TLR2 ligands was not affected.
Materials and methods
Patients
Blood was collected from 74 patients with Crohn’s disease. Polymerase chain reaction amplification of NOD2 gene fragments containing the polymorphic site 3020insC was performed in 50-μl reaction volumes containing 100–200 ng genomic DNA, as previously described.8 The 3020insC polymorphism was analysed by Genescan analysis on an ABI Prism 3100 Genetic Analyzer according to the protocol of the manufacturer (Applied Biosystems, Nieuwerkerk a/d IJssel, the Netherlands). Five patients identified as homozygous for the 3020insC NOD2 allele were included in the study. From the patients with Crohn’s disease who were homozygous for the wild-type NOD2 allele, five were recruited as a control group, in addition to five healthy volunteers with the wild-type allele. None of the patients had active disease at the time of blood donation, and none had used corticosteroids or other anti-inflammatory medication for at least 2 weeks prior to the experiment.
Isolation of mononuclear cells and cross-tolerance
Blood from patients and volunteers was drawn into ethylenediaminetetraacetic acid tubes, and isolation of mononuclear cells was performed as described elsewhere.17 Mononuclear cells (5 × 105 in 100 μl) were added to round-bottom 96-well plates and preincubated for 24 hr with either 100 μl culture medium (negative control), or MDP at concentrations 0·1, 1 or 10 μg/ml. After incubation for a further 24 hr, the supernatant was discarded, the adherent cells were washed, and restimulation with various stimuli was performed: control medium, highly purified Salmonella typhimurium LPS (1 ng/ml), synthetic Pam3Cys or macrophage-activating lipopeptide 2 (MALP) (both at 10 μg/ml), or heat-killed S. typhimurium or Bacteroides fragilis (both at a concentration of 1 × 106 microorganisms/ml). Enzyme-linked immunosorbent assay was used to measure TNF, IL-6, IL-10 and IL-12 in the supernatants (Pelikine Compact, Sanquin, the Netherlands).
Immunohistochemistry of intestinal wall peptidoglycans
After obtaining informed consent, colonic biopsies were taken from three patients undergoing routine follow-up colonoscopy for colonic polyps, and three patients with Crohn’s disease bearing the wild-type NOD2 allele who were undergoing a routine check-up. Tissue samples were fixed with 4% formaldehyde. After 2 hr the specimens were transferred to a solution of 70% alcohol, and then embedded in paraffin. After dewaxing and dehydration, sections were blocked with normal swine serum followed by 60 min of incubation with a monoclonal mouse anti-MDP antibody (kindly provided by Dr George M. Bahr, Pasteur Institute, Lille, France) at 0·3 μg/ml. The secondary antibody, biotinylated rabbit anti-mouse immunoglobulin, was added to the incubation for 30 min. Slides were stained with streptavidin peroxidase, developed with diaminobenzidine (DAB), and counterstained with haematoxylin for 30 seconds.
Statistical analysis
Experiments were performed in duplicate. Data are given as mean ± SD. The differences between groups were analysed by Mann–Whitney U-test, and where appropriate by paired Wilcoxon test. The level of significance was set at P < 0·05.
Results
NOD2-induced cross-tolerance for TLR4-induced TNF-α
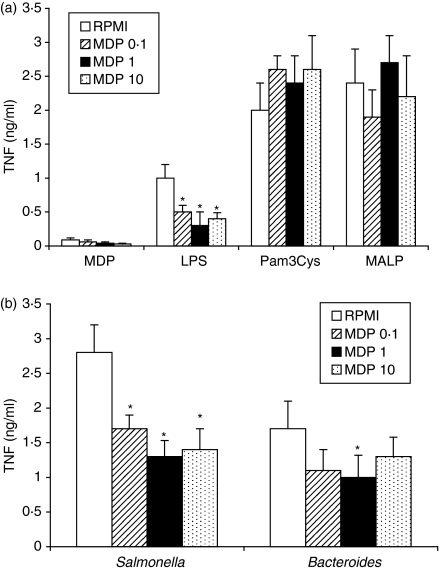
Prestimulation of peripheral blood mononuclear cells (PBMC) isolated from healthy volunteers with various concentrations of MDP significantly reduced their capacity to synthesize TNF-α after stimulation of TLR4 by LPS, but not after stimulation with the TLR2 ligands Pam3Cys or MALP (Fig. 1a). To investigate whether MDP can also induce tolerance to intestinal pathogens, cells exposed to MDP were subsequently stimulated with heat-killed S. typhimurium or B. fragilis. Although both are intestinal microorganisms, S. typhimurium induces intracellular signals through both TLR4 and TLR2,18 whereas TLR2 is the main receptor mediating the cytokine production induced by B. fragilis.19 In line with the results obtained with purified TLR ligands, stimulation of NOD2 by MDP led to tolerance to the induction of TNF-α by S. typhimurium (Fig. 1b). The effect of NOD2 engagement was less pronounced on the stimulation of cytokines by B. fragilis (Fig. 1b).
Figure 1.
NOD2 induces cross-tolerance for TLR4-induced TNF-α. (a) Mononuclear cells isolated from five healthy volunteers were preincubated for 24 hr with various concentrations of MDP (0·1, 1 and 10 μg/ml), and subsequently stimulated for 24 hr with LPS (1 ng/ml), Pam3Cys (10 μg/ml) or MALP (10 μg/ml). (b) To investigate whether MDP can also induce tolerance to stimulation with intestinal pathogens, mononuclear cells tolerized with MDP in various concentrations (see above) were subsequently stimulated with heat-killed S. typhimurium or B. fragilis (both at a concentration of 1 × 106 microorganisms/ml). Concentrations of TNF-α were measured by enzyme-linked immunosorbent assay. Data are presented as mean ± SD, and compared by Wilcoxon paired test (n = 5, *P < 0·05).
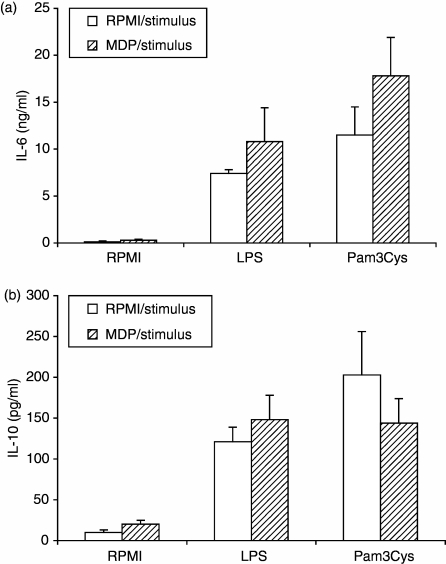
In contrast to TNF production, the synthesis of IL-6 in response to the various stimuli was not changed by the preincubation of PBMC with MDP (Fig. 2). Similarly, IL-10 production induced by TLR2 or TLR4 ligands was not changed by preincubation of PBMC with MDP (Fig. 2). Production of IL-12 was undetectable in all the samples, irrespective of the stimulus used.
Figure 2.
NOD2 does not influence the induction of IL-6 and IL-10 stimulated by TLRs. MNC isolated from five healthy volunteers were preincubated for 24 hr with MDP (10 μg/ml), and subsequently stimulated for another 24 hr with LPS (1 ng/ml) or Pam3Cys (10 μg/ml). Concentrations of IL-6 (a) and IL-10 (b) were measured by enzyme-linked immunosorbent assay. Data are presented as mean ± SD, and compared by Wilcoxon paired test (n = 5, all differences not significant).
Presence of bacterial peptidoglycans in the intestinal wall
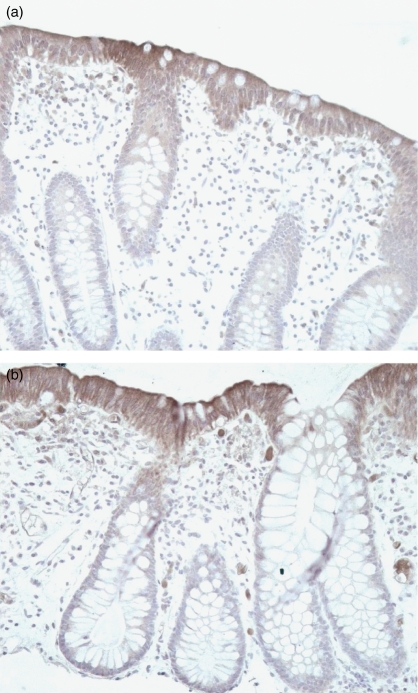
The hypothesis that NOD2 signals induce tolerance for subsequent stimulation of TLR would require the presence of sufficient amounts of peptidoglycan in enterocytes. Indeed, immunostaining for peptidoglycans clearly demonstrated the intracellular presence of peptidoglycan in colonic enterocytes, both in healthy individuals (Fig. 3a) and in patients with Crohn’s disease (Fig. 3b).
Figure 3.
Peptidoglycan is present in the intestinal mucosa. Immunostaining using a monoclonal mouse anti-MDP antibody (0·3 μg/ml) was performed in the colonic mucosa of healthy individuals (a) and patients with Crohn’s disease (b). The secondary antibody, biotinylated rabbit anti-mouse immunoglobulin G, was added to the incubation for 30 min. Slides were stained with streptavidin peroxidase, developed with DAB, and counterstained with haematoxylin for 30 seconds (magnification 400×).
NOD2/TLR4 cross-tolerance in Crohn’s disease patients with a 3020insC NOD2 mutation
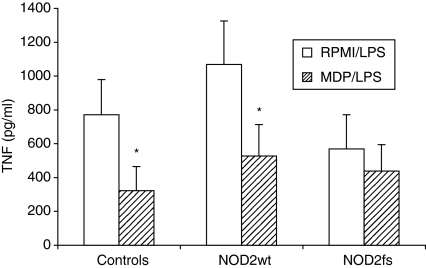
NOD2-induced cross-tolerance on TLR4-mediated TNF-α production was investigated in volunteers, Crohn’s disease patients with the wild-type NOD2 allele (NOD2wt), and patients who were homozygous for the 3020insC mutation (NOD2fs). Cross-tolerance between NOD2 and TLR4 was only absent in patients with the NOD2fs mutation (Fig. 4).
Figure 4.
Cross-tolerance induced by NOD2 stimulation is absent in patients with the 3020insC mutation. Cross-tolerance between NOD2 and TLR4, as measured by the effect of MDP (10 μg/ml) preincubation on LPS-induced (1 ng/ml) TNF-α production, was assessed in five healthy controls, five Crohn’s patients bearing the wild-type allele (NOD2wt), and five patients with the 3020insC NOD2 mutation (NOD2fs). Data are presented as mean ± SD, and compared by Mann–Whitney test (n = 5, *P < 0·05).
Discussion
In this study we have demonstrated that stimulation of NOD2 by MDP induces tolerance to subsequent induction of TLR4 by intestinal microorganisms and other ligands. This tolerance affects the production of TNF-α, a proinflammatory cytokine with a central role in the pathogenesis of Crohn’s disease.20 Neutralization of TNF by monoclonal antibodies has been proved to be the most effective immunotherapy for Crohn’s disease.21 The observed tolerance may represent an important mechanism allowing the presence of colonizing intestinal flora without activating the immune system. The absence of such tolerization in individuals with NOD2 mutations predisposes to excessive inflammation, and is likely to represent a key factor in the increased susceptibility of these individuals to develop Crohn’s disease.
Several mechanisms have been proposed to explain the correlation between loss-of-function mutations in NOD2 and Crohn’s disease. We previously proposed that defective synthesis of the anti-inflammatory cytokine IL-10, known to protect against experimental colitis,22 is important for the development of Crohn’s disease. Others have proposed that defective barrier defence, through ineffective production of defensins at the level of the intestinal mucosa, could explain bacterial persistence and the induction of inflammation.10,11 Other hypotheses included NOD2-dependent inhibition of TLR2 signalling,23 or NOD2 3020insC gain-of-function effects on IL-1β release in a mouse model,12 but these two mechanisms could not be confirmed in subsequent studies. Instead, several studies in human primary cells demonstrated synergism between NOD2 and TLR2 stimulation, whereas monocytes isolated from NOD2 3020insC homozygous patients demonstrated defective IL-1β production.9,13,14 Thus, none of these mechanisms adequately explains the excessive inflammation found in Crohn’s disease. Although other pathways may also be involved, we propose that intestinal inflammation in the mucosa of these patients is the result of the absence of NOD2/TLR4 cross-tolerance, leading to subsequent TNF-α production induced by intestinal flora triggering TLR4. Although patients with Crohn’s disease bearing the NOD2 mutations had a slightly (not significant) lower TNF release compared with the other two groups, they were completely refractory to the NOD2-induced cross-tolerance for LPS stimulation. However, we have to underline that the present study has been performed only with circulating monocytes. Lamina propria monocytes and enterocytes have been reported to have a more tolerant phenotype, and the NOD2/TLR cross-tolerance experiments from the present study should be repeated in lamina propria cells.
Marks et al. recently proposed an intriguing hypothesis for the pathogenesis of Crohn’s disease, demonstrating that patients with Crohn’s disease have defective production of proinflammatory cytokines, including IL-8, and deficient neutrophil recruitment.24 This immunodeficient state may lead to ineffective bacterial clearance from the intestinal wall, with subsequent inflammation. However, these defects were reported for Crohn’s disease patients irrespective of their NOD2 make-up. Our study presents evidence for a ‘second hit’ mechanism in patients bearing NOD2 mutations. Healthy individuals with wild-type NOD2 can mount a proper IL-1β and IL-8 response after challenge with NOD2 ligands24 enabling elimination of invading organisms, and have an attenuated TNF-α release because of the NOD2/TLR4 cross-tolerance. In contrast, patients with Crohn’s disease with wild-type NOD2 have an intrinsic IL-8 defect, but this defect can be partly overcome by MDP–NOD2 ligation.24 However, patients with Crohn’s disease and the 3020insC NOD2 mutation have both a deficient IL-8 response, leading to persistence of microbial stimuli, and respond with an abundant TNF-α response to intestinal flora because of absence of NOD2/TLR4 cross-tolerance.
The relevance of our data for the pathogenesis of Crohn’s is supported by two additional lines of evidence: first, we demonstrated the presence of intracellular peptidoglycans in enterocytes, enabling prolonged interaction with NOD2 and induction of tolerance; and second, tolerance was not only demonstrated for LPS but also for whole intestinal microorganisms. Finally, it should be noted that NOD2 induces specific tolerance for TLR4 stimulation, but not that by TLR2. While both TLR2 and TLR4 mediate nuclear factor-κB translocation and TNF transcription through the myeloid differentiation factor 88 (MyD88)-dependent pathway, in the case of TLR4 (but not TLR2) a second pathway of nuclear factor-κB/TNF induction involves TIR domain-containing adaptor-inducing interferon-β (TRIF)/TRIF-related adaptor molecule (TRAM)-dependent signalling. Thus, one may speculate that it is the TRIF/TRAM TLR4-specific pathway that is modulated by NOD2, although additional experiments are needed to confirm that. In addition, several studies have now shown that TLR4 primarily induces strong proinflammatory signals, whereas TLR2 predominantly provokes an anti-inflammatory profile (reviewed in ref. 25). Thus, whereas NOD2 induces cross-tolerance against proinflammatory TLR4 signals, it preserves the attenuating TLR2 stimulation. In line with this, only LPS-induced production of TNF, a strong proinflammatory cytokine, has been inhibited by preincubation of cells with MDP, whereas production of the anti-inflammatory cytokines IL-10 and IL-6 was not affected. This demonstrates a specific cross-tolerance effect between NOD2 and TLR4 on TNF release, resulting in a net proinflammatory bias in patients lacking a functional NOD2.
In conclusion, we provide in this study the proof-of-concept that NOD2 can specifically induce cross-tolerance to TNF-α stimulation by TLR4 ligands and intestinal microorganisms. This may represent an important mechanism through which overwhelming inflammatory responses to intestinal flora are prevented. However, these effects should be validated in future experiments on lamina propria cells. In addition, additional studies on the precise molecular mechanism of the cross-tolerance are also warranted. This protective cross-tolerance mechanism is absent in patients with Crohn’s disease who are homozygous for the 3020insC NOD2 mutation, and this may contribute to their susceptibility to Crohn’s disease.
Acknowledgments
M.G.N. was supported by a Vidi-grant from the Netherlands Organization for Scientific Research (NWO-ZonMW).
Abbreviations
- IL
interleukin
- MDP
muramyl dipeptide
- NOD2
nucleotide oligomerization domain-2
- TLR4
Toll-like receptor 4
- TNF-α
tumour necrosis factor-α
References
- 1.Hugot J-P, Laurent-Puig P, Gower-Rousseau C, et al. Mapping of a susceptibility locus for Crohn’s disease on chromosome 16. Nature. 1996;379:821–3. doi: 10.1038/379821a0. [DOI] [PubMed] [Google Scholar]
- 2.Hugot J-P, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 3.Hampe J, Grebe J, Nikolaus S, et al. Association of NOD2 (CARD15) genotype with clinical course of Crohn’s disease: a cohort study. Lancet. 2002;359:1661–5. doi: 10.1016/S0140-6736(02)08590-2. [DOI] [PubMed] [Google Scholar]
- 4.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez O, Pipaon C, Inohara N, et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-κB activation. J Biol Chem. 2002;277:41701–5. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 6.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 7.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through Nod2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–12. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 8.Netea MG, Kullberg BJ, de Jong D, et al. NOD2 mediates induction of the antiinflammatory signals induced by TLR2-ligands: implications for Crohn’s disease. Eur J Immunol. 2004;34:2052–9. doi: 10.1002/eji.200425229. [DOI] [PubMed] [Google Scholar]
- 9.van Heel DA, Ghosh S, Hunt K, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn’s disease. Lancet. 2005;365:1794–6. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 10.Wehkamp J, Harder J, Weichenthal M, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–64. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi KS, Chamaillard M, Ogura Y, et al. NOD2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 12.Maeda S, Hsu L-C, Liu H, et al. NOD2 mutation in Crohn’s disease potentiates NF-κB actvity and IL-1β processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 13.Netea MG, Ferwerda G, de Jong DJ, Girardin SE, Kullberg BJ, van der Meer JW. NOD2 3020insC mutation and the pathogenesis of Crohn’s disease: impaired IL-1beta production points to a loss-of-function phenotype. Neth J Med. 2005;63:305–8. [PubMed] [Google Scholar]
- 14.Netea MG, Ferwerda G, De Jong DJ, et al. NOD2 modulates specific Toll-like receptor pathways for the induction of cytokine release. J Immunol. 2005;174:6518–23. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 15.Sato S, Nomura F, Kawai T, et al. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 16.Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166:5161–7. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 17.Endres S, Cannon JG, Dempsey RA, et al. In vitro production of IL-1β, IL-1α, TNF and IL-2 in healthy subjects: distribution, effect of oral cyclooxygenase inhibitors and evidence of independent gene regulation. Eur J Immunol. 1989;19:2327–33. doi: 10.1002/eji.1830191222. [DOI] [PubMed] [Google Scholar]
- 18.Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol. 2000;165:5780–7. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- 19.Erridge C, Pridmore A, Eley A, Stewart J, Poxton IR. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via toll-like receptor 2. J Med Microbiol. 2004;53:735–40. doi: 10.1099/jmm.0.45598-0. [DOI] [PubMed] [Google Scholar]
- 20.van Deventer SJ. Targeting TNF alpha as a key cytokine in the inflammatory processes of Crohn’s disease – the mechanisms of action of infliximab. Aliment Pharmacol Ther. 1999;13(Suppl. 4):3–8. doi: 10.1046/j.1365-2036.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 21.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 22.Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143–9. [PubMed] [Google Scholar]
- 23.Watanabe K, Kagaya K, Yamada T, Fukazawa Y. Mechanism for candidacidal activity in macrophages activated by recombinant gamma-interferon. Infect Immun. 1991;59:521–8. doi: 10.1128/iai.59.2.521-528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks DJ, Harbord MW, MacAllister R, et al. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367:668–78. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 25.Netea MG, Van der Meer JW, Sutmuller RP, Adema GJ, Kullberg BJ. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrob Agents Chemother. 2005;49:3991–6. doi: 10.1128/AAC.49.10.3991-3996.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]