Abstract
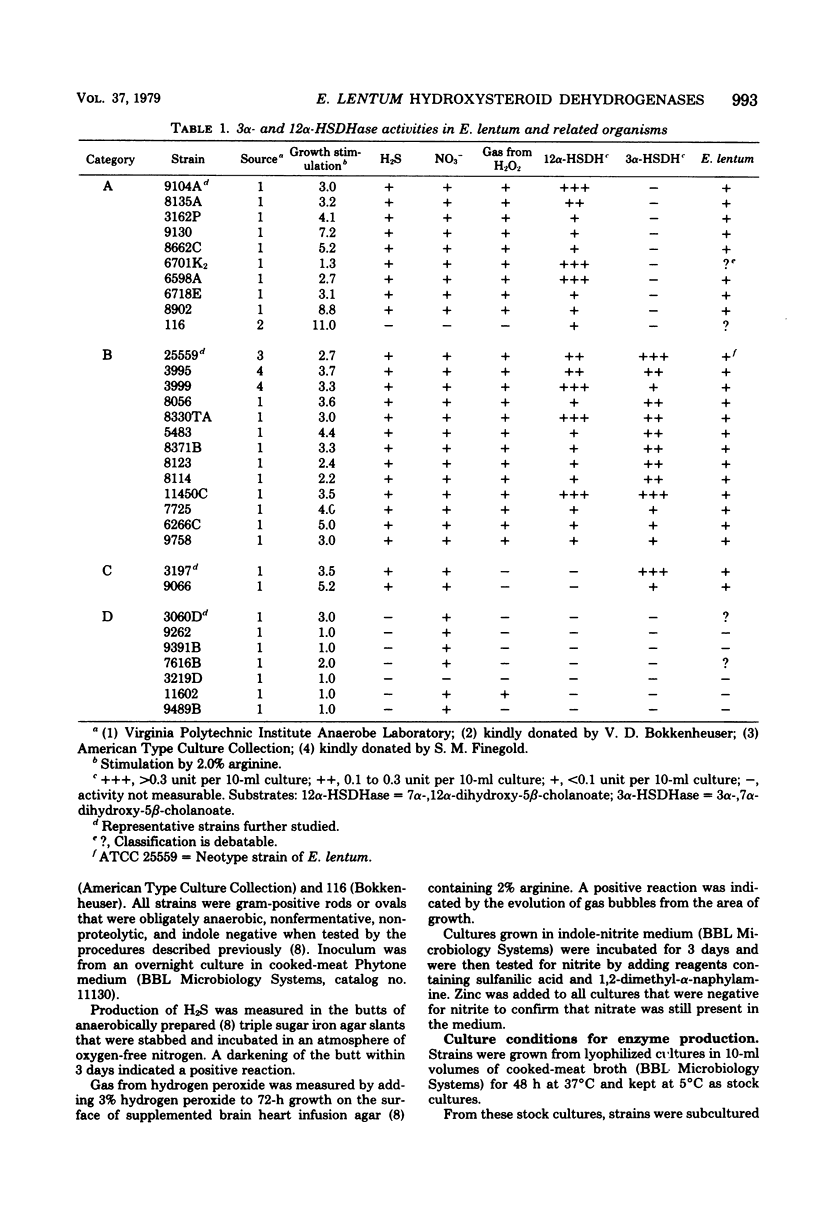
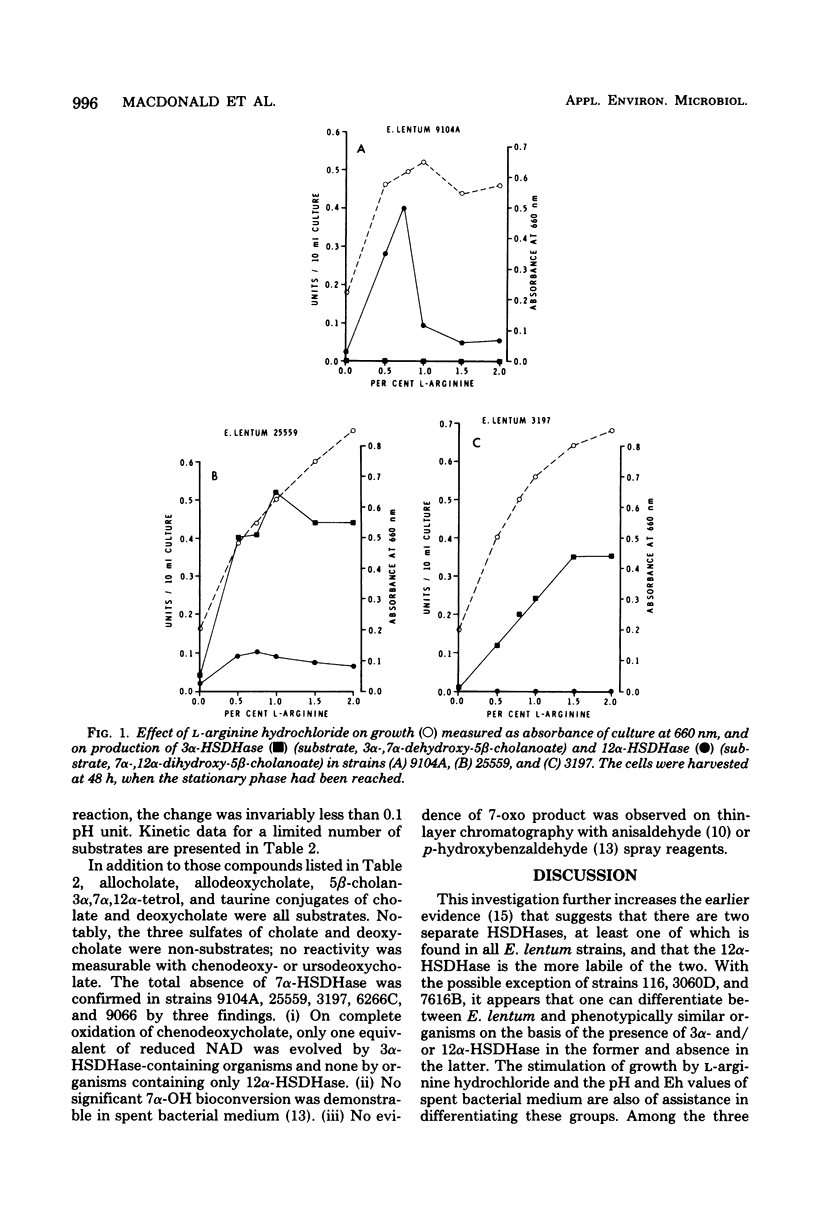
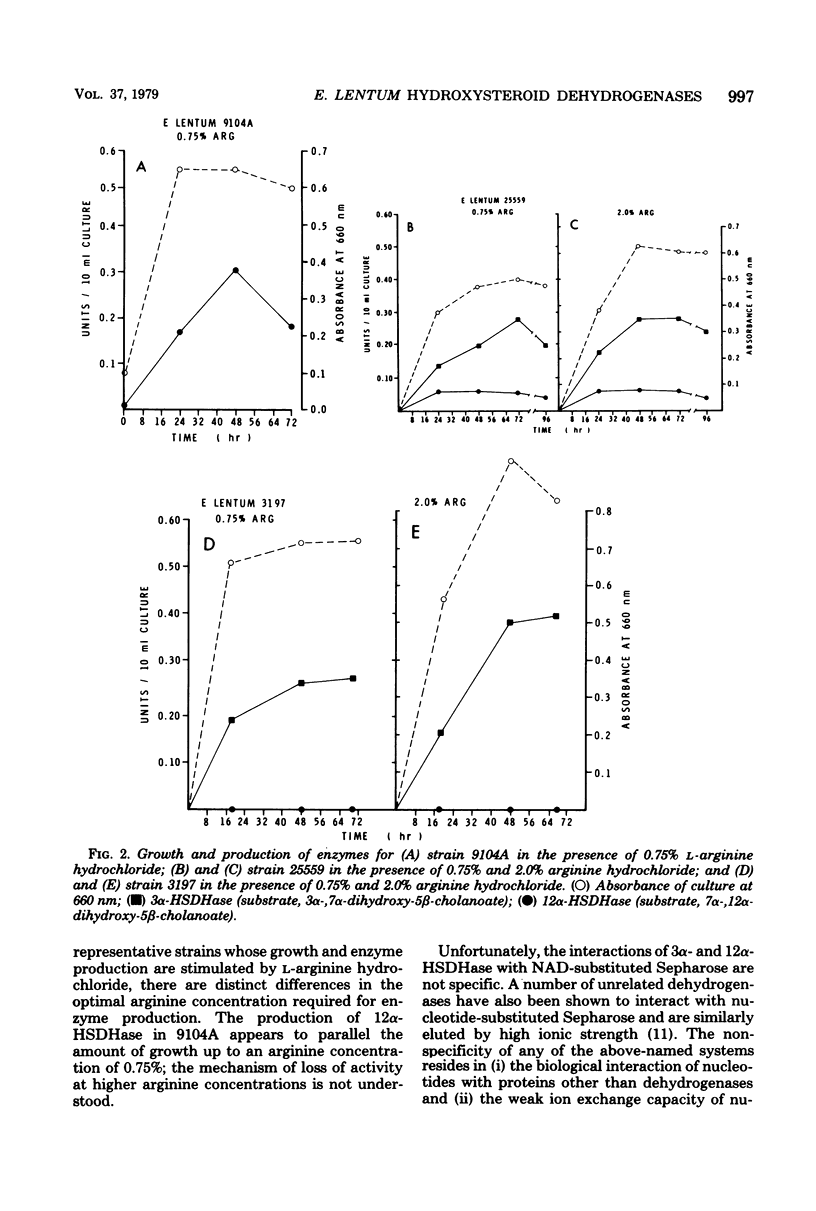
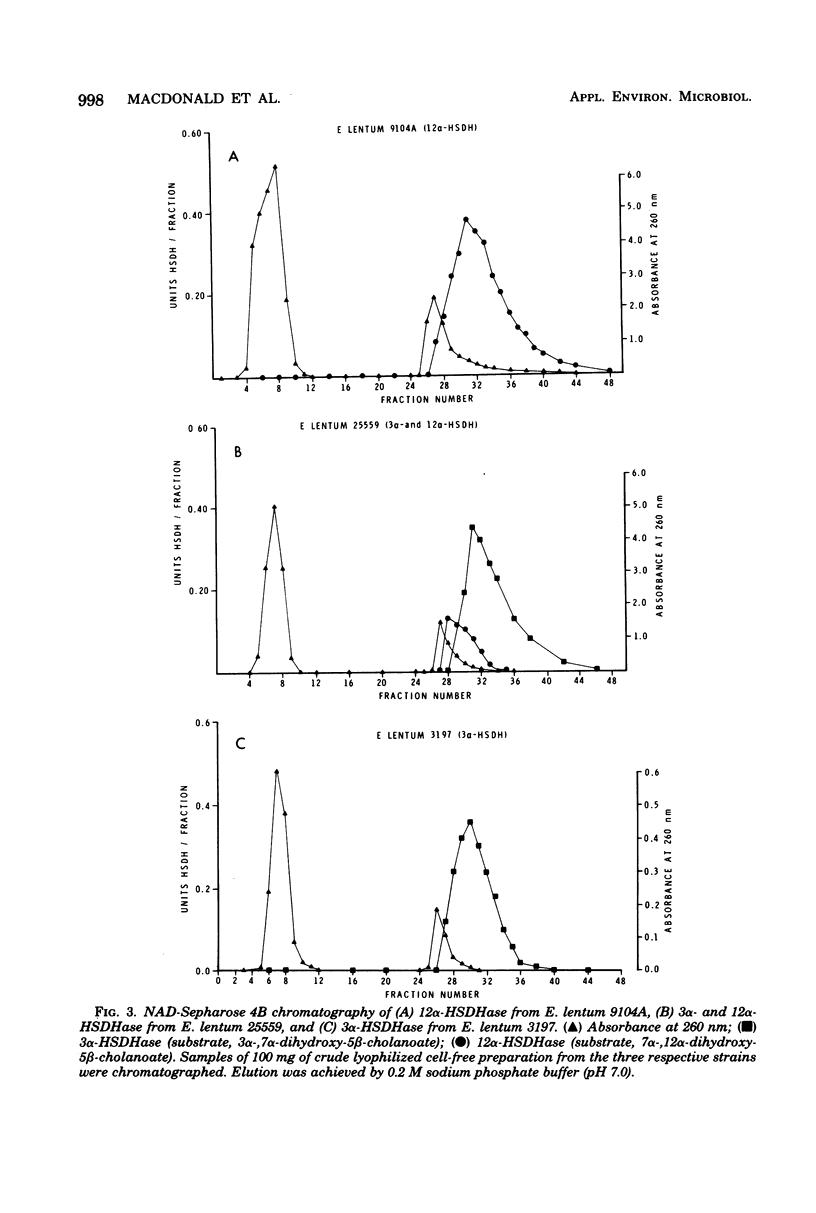
Thirty-two strains of Eubacterium lentum and phenotypically similar anaerobic gram-positive bacilli were screened for intracellular bile salt 3α- and 12α-hydroxysteroid dehydrogenase (HSDHase) activities. These organisms were categorized into four groups: (A) those containing 12α-HSDHase only (10 strains), (B) those containing 3α- and 12α-HSDHase (13 strains), (C) those containing 3α-HSDHase only (2 strains), and (D) those devoid of any measurable HSDHase activity (7 strains). Of the respective four groups, 9/10, 13/13, 0/2, and 0/7 were like the neotype strain of E. lentum (ATCC 25559) in that they produced H2S in a triple sugar iron agar butt, reduced nitrate to nitrite, and weakly decomposed hydrogen peroxide. The other strains were variable for nitrate reduction and activity on hydrogen peroxide, but all the organisms in the first three categories (with one exception) were H2S producers (triple sugar iron agar butt) and all (with one exception) were designated E. lentum, whereas the organisms of category B were non-H2S producers (triple sugar iron agar butt). Five of these seven were not stimulated by arginine and are designated “phenotypically similar organisms.” Thin-layer chromatography of extracted spent bacterial medium of four representative strains from each group grown in the presence of cholate revealed the presence of (A) 12-oxo product, (B) 12-oxo and 3-oxo products, (C) 3-oxo product, and (D) the absence of any of these products. The 12α-HSDHase of category B organisms was unstable unless 10−3 M dithioerythritol was added to the buffer. With the exception of 3 out of 32 strains, there was a positive correlation between the production of measurable amounts of 12α-HSDHase and H2S production. Growth curves and the effect of arginine on growth and the production of 3α- and 12α-HSDHase were examined in representative strains of categories A, B, and C. Both enzymes were shown to bind onto a nicotinamide adenine dinucleotide-Sepharose column and could be eluted by high-ionic-strength buffer, resulting in approximately 25-fold and 18-fold purification, respectively. Molecular weight estimations by Sephadex G-200 gave values of 205,000 and 125,000 for the 3α- and 12α-HSDHase, respectively. Purified 12α-HSDHase was investigated with respect to pH requirement, substrate specificity, and enzyme kinetics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bokkenheuser V. D., Winter J., Dehazya P., Kelly W. G. Isolation and characterization of human fecal bacteria capable of 21-dehydroxylating corticoids. Appl Environ Microbiol. 1977 Nov;34(5):571–575. doi: 10.1128/aem.34.5.571-575.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Haslewood E. S., Haslewood G. A. The specificity of a 7 alpha-hydroxy steroid dehydrogenase from Escherichia coli. Biochem J. 1976 Jul 1;157(1):207–210. doi: 10.1042/bj1570207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWATA T., YAMASAKI K. ENZYMATIC DETERMINATION AND THIN-LAYER CHROMATOGRAPHY OF BILE ACIDS IN BLOOD. J Biochem. 1964 Nov;56:424–431. doi: 10.1093/oxfordjournals.jbchem.a128013. [DOI] [PubMed] [Google Scholar]
- KRITCHEVSKY D., MARTAK D. S., ROTHBLAT G. H. Detection of bile acids in thin-layer chromatography. Anal Biochem. 1963 May;5:388–392. doi: 10.1016/0003-2697(63)90040-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacDonald I. A., Mahony D. E., Jellet J. F., Meier C. E. NAD-dependent 3alpha- and 12alpha-hydroxysteroid dehydrogenase activities from Eubacterium lentum ATCC no. 25559. Biochim Biophys Acta. 1977 Dec 21;489(3):466–476. doi: 10.1016/0005-2760(77)90167-9. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A. Detection of bile salts with Komarowsky's reagent and group specific dehydrogenases. J Chromatogr. 1977 Jun 11;136(2):348–352. doi: 10.1016/s0021-9673(00)86292-5. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Jellett J. F., Mahony D. E. 12alpha-Hydroxysteroid dehydrogenase from Clostridium group P strain C48-50 ATCC No. 29733: partial purification and characterization. J Lipid Res. 1979 Feb;20(2):234–239. [PubMed] [Google Scholar]
- Macdonald I. A., Williams C. N., Mahony D. E. Behavior of 3alpha- and 7alpha-hydroxysteroid dehydrogenases on chenodeoxycholate substituted Sepharose. Steroids. 1976 Jul;28(1):25–30. doi: 10.1016/0039-128x(76)90122-7. [DOI] [PubMed] [Google Scholar]
- Mahony D. E., Meier C. E., Macdonald I. A., Holdeman L. V. Bile salt degradation by nonfermentative clostridia. Appl Environ Microbiol. 1977 Oct;34(4):419–423. doi: 10.1128/aem.34.4.419-423.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQIRE P. G., DELIN S., PORATH J. PHYSICAL AND CHEMICAL CHARACTERIZATION OF HYDROXYSTEROID DEHYDROGENASES FROM PSEUDOMONAS TESTOSTERONI. Biochim Biophys Acta. 1964 Sep 18;89:409–421. doi: 10.1016/0926-6569(64)90067-7. [DOI] [PubMed] [Google Scholar]
- Sperry J. F., Wilkins T. D. Arginine, a growth-limiting factor for Eubacterium lentum. J Bacteriol. 1976 Aug;127(2):780–784. doi: 10.1128/jb.127.2.780-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry J. F., Wilkins T. D. Cytochrome spectrum of an obligate anaerobe, Eubacterium lentum. J Bacteriol. 1976 Mar;125(3):905–909. doi: 10.1128/jb.125.3.905-909.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


