Abstract
Alcohol dehydrogenase of Rhizopus javanicus was purified, and its physical and chemical characteristics were determined. The intact enzyme was shown to have a molecular weight of approximately 60,000. Since the smallest apparent subunit was 14,000, the enzyme was presumed to be composed of four subunits. The crude mycelial extract contained multiple forms of the enzyme, which were separated by ion-exchange chromatography.
Full text
PDF
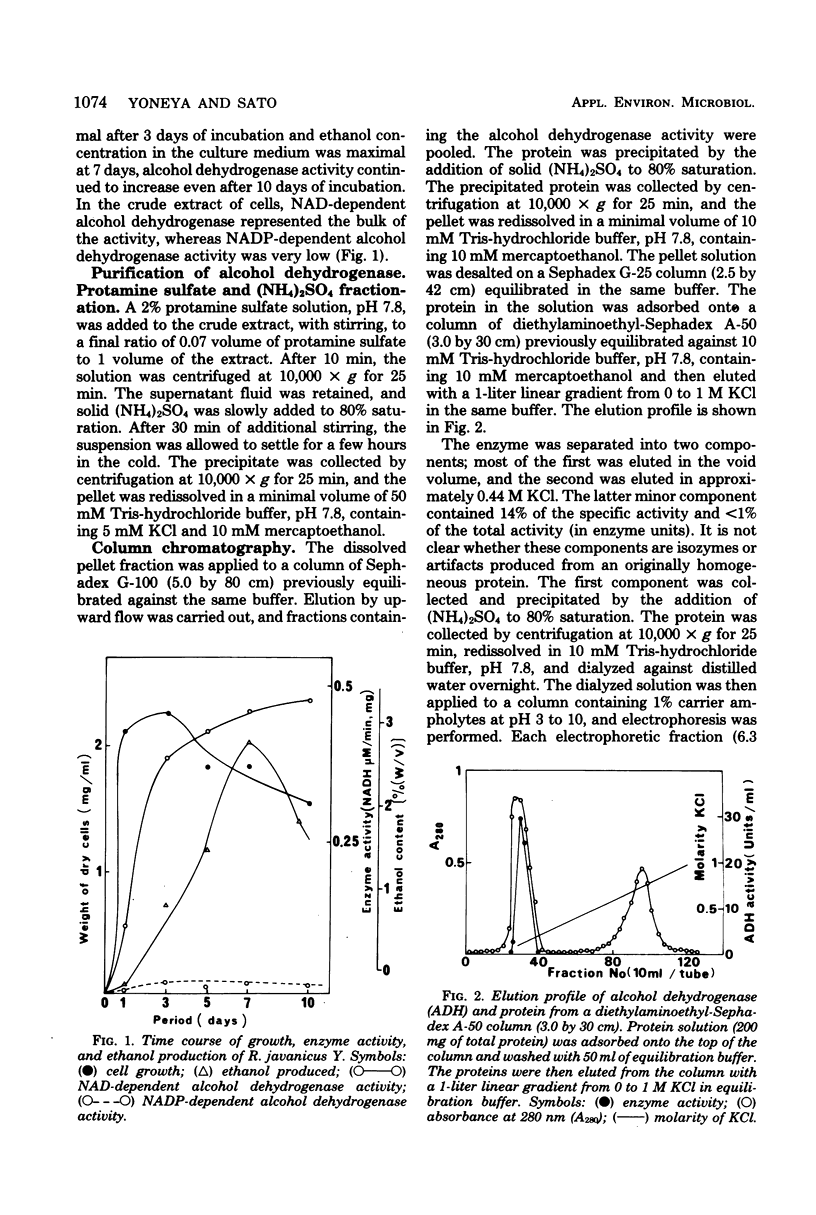
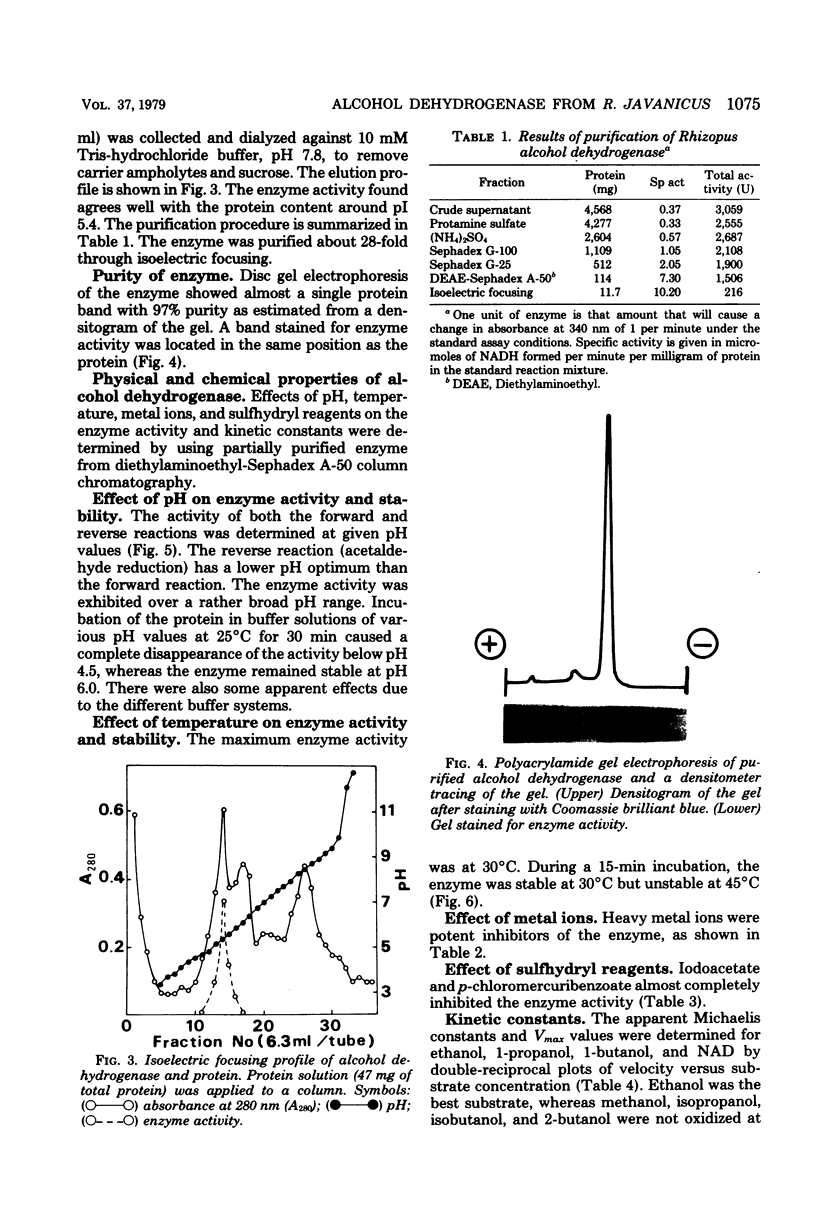
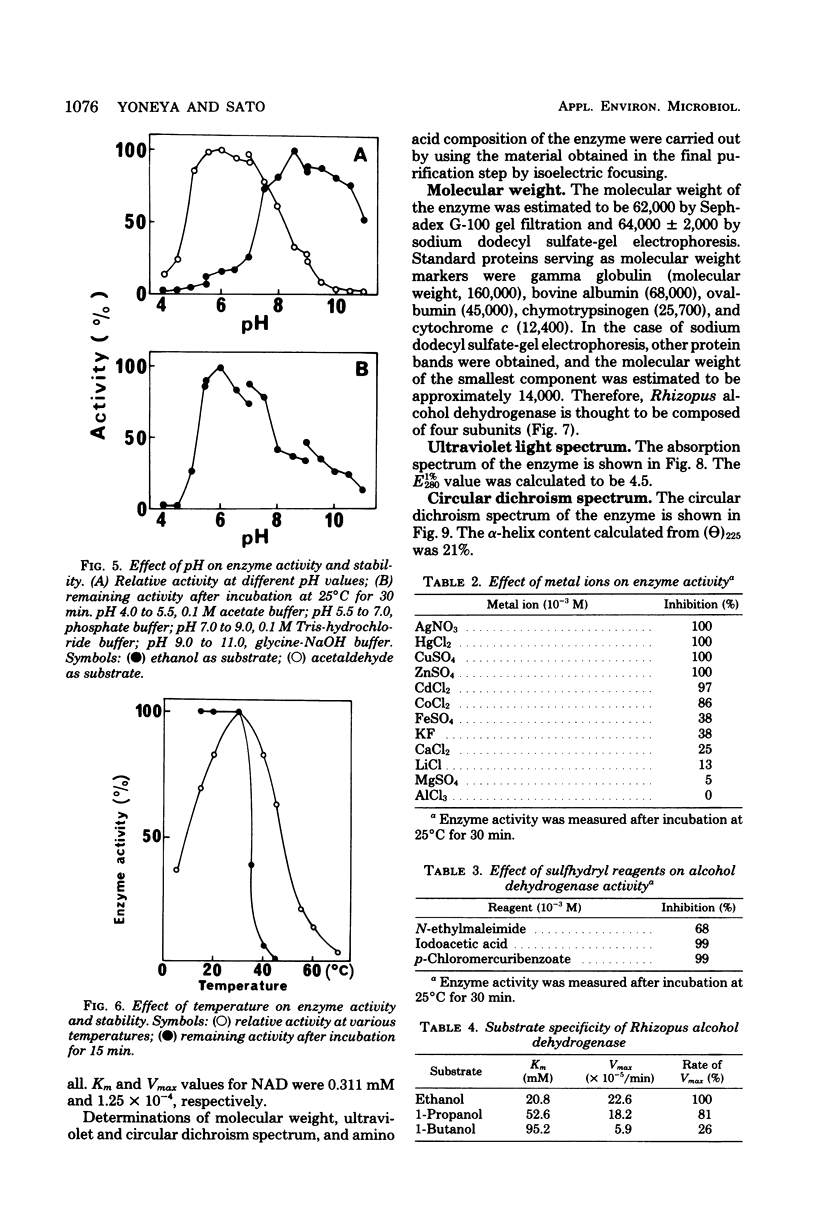

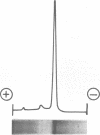
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Felder M. R., Scandalios J. G., Liu E. H. Purification and partial characterization of two genetically defined alcohol dehydrogenase isozymes in maize. Biochim Biophys Acta. 1973 Jul 12;317(1):149–159. doi: 10.1016/0005-2795(73)90207-9. [DOI] [PubMed] [Google Scholar]
- Heick H. M., Willemot J., Begin-Heick N. The subcellular localization of alcohol dehydrogenase activity in baker's yeast. Biochim Biophys Acta. 1969;191(3):493–501. doi: 10.1016/0005-2744(69)90342-8. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Pfuderer P. Interconversion of isoenzymes of Drosophila alcohol dehydrogenase. II. Physical characterization of the enzyme and its subunits. J Biol Chem. 1970 Aug 10;245(15):3938–3944. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sofer W., Ursprung H. Drosophila alcohol dehydrogenase. Purification and partial characterization. J Biol Chem. 1968 Jun 10;243(11):3110–3115. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woscinski C. J., McClary D. O. Alcohol dehydrogenase activity in Rhodotorula glutinis. Can J Microbiol. 1973 Mar;19(3):353–358. doi: 10.1139/m73-058. [DOI] [PubMed] [Google Scholar]