Abstract
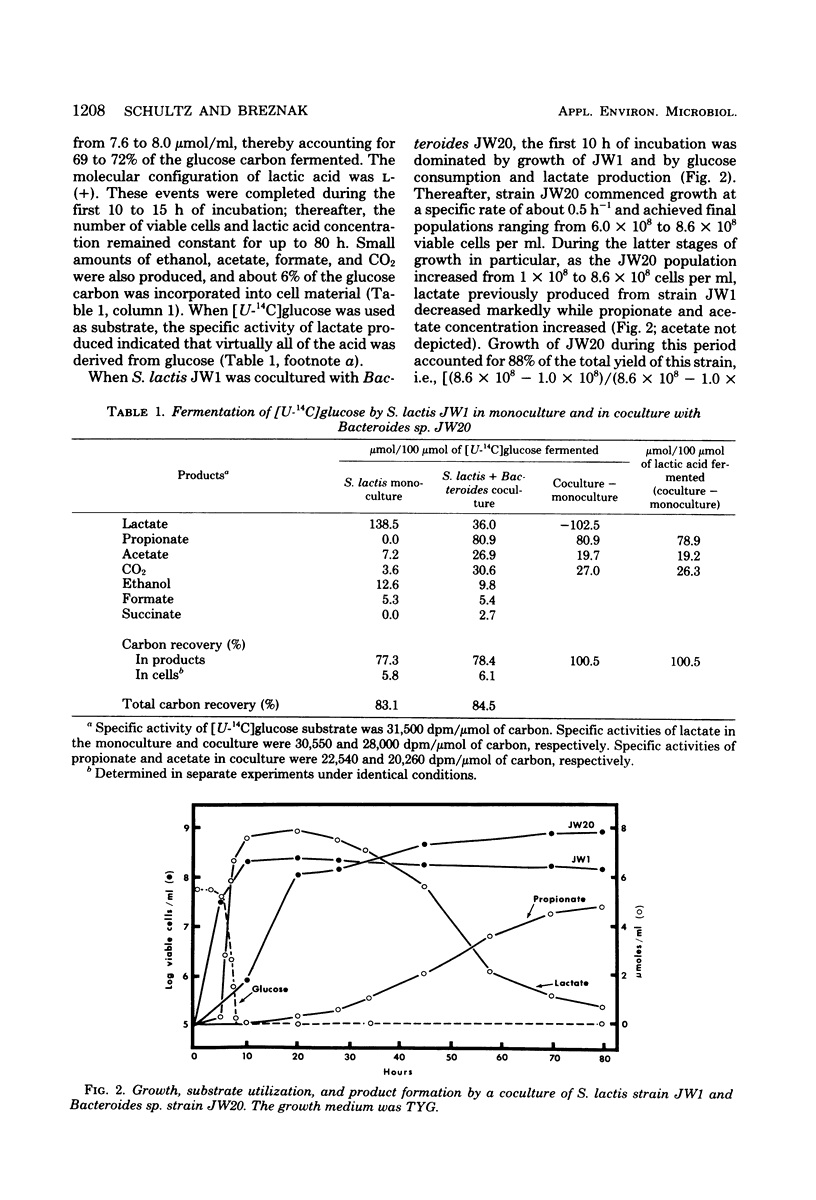
Streptococcus lactis and Bacteroides sp., isolated from hindguts of Reticulitermes flavipes termites, were grown anaerobically in monoculture and coculture. When grown in a glucose medium, S. lactis monoculture produced lactate as the major fermentation product, with small amounts of formate, acetate, ethanol, and CO2. In coculture, glucose was completely consumed during growth of S. lactis. Lactate, produced by S. lactis, then supported much of the growth of Bacteroides and was fermented to propionate, acetate, and CO2. Small amounts of succinate were formed during growth of Bacteroides in the coculture, but little change in the formate or ethanol concentration was observed. Monoculture growth of Bacteroides in a tryptone-yeast extract medium revealed that incorporation of 20 to 40 mM lactate increased cell yields and production of organic acids. However, initial lactate concentrations greater than 40 mM suppressed not only growth of Bacteroides but also acidic product formation. Results suggest that cross-feeding of lactate between streptococci and bacteroides constitutes one aspect of the overall hindgut fermentation in termites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Lysons R. J., Alexander T. J., Wellstead P. D., Hobson P. N., Mann S. O., Stewart C. S. Defined bacterial populations in the rumens of gnotobiotic lambs. J Gen Microbiol. 1976 Jun;94(2):257–269. doi: 10.1099/00221287-94-2-257. [DOI] [PubMed] [Google Scholar]
- Macy J. M., Ljungdahl L. G., Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. J Bacteriol. 1978 Apr;134(1):84–91. doi: 10.1128/jb.134.1.84-91.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikx F. H., Van der Hoeven J. S. Symbiosis of Streptococcus mutans and Veillonella alcalescens in mixed continuous cultures. Arch Oral Biol. 1975 Jul;20(7):407–410. doi: 10.1016/0003-9969(75)90224-1. [DOI] [PubMed] [Google Scholar]
- Ng S. K., Hamilton I. R. Lactate metabolism by Veillonella parvula. J Bacteriol. 1971 Mar;105(3):999–1005. doi: 10.1128/jb.105.3.999-1005.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Nitrogen-fixing Enterobacter agglomerans isolated from guts of wood-eating termites. Appl Environ Microbiol. 1977 Feb;33(2):392–399. doi: 10.1128/aem.33.2.392-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Breznak J. A. Heterotrophic bacteria present in hindguts of wood-eating termites [Reticulitermes flavipes (Kollar)]. Appl Environ Microbiol. 1978 May;35(5):930–936. doi: 10.1128/aem.35.5.930-936.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallnöfer P., Baldwin R. L. Pathway of propionate formation in Bacteroides ruminicola. J Bacteriol. 1967 Jan;93(1):504–505. doi: 10.1128/jb.93.1.504-505.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]