Abstract
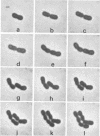
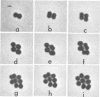
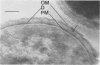
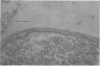
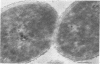
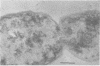
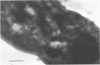
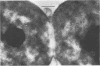
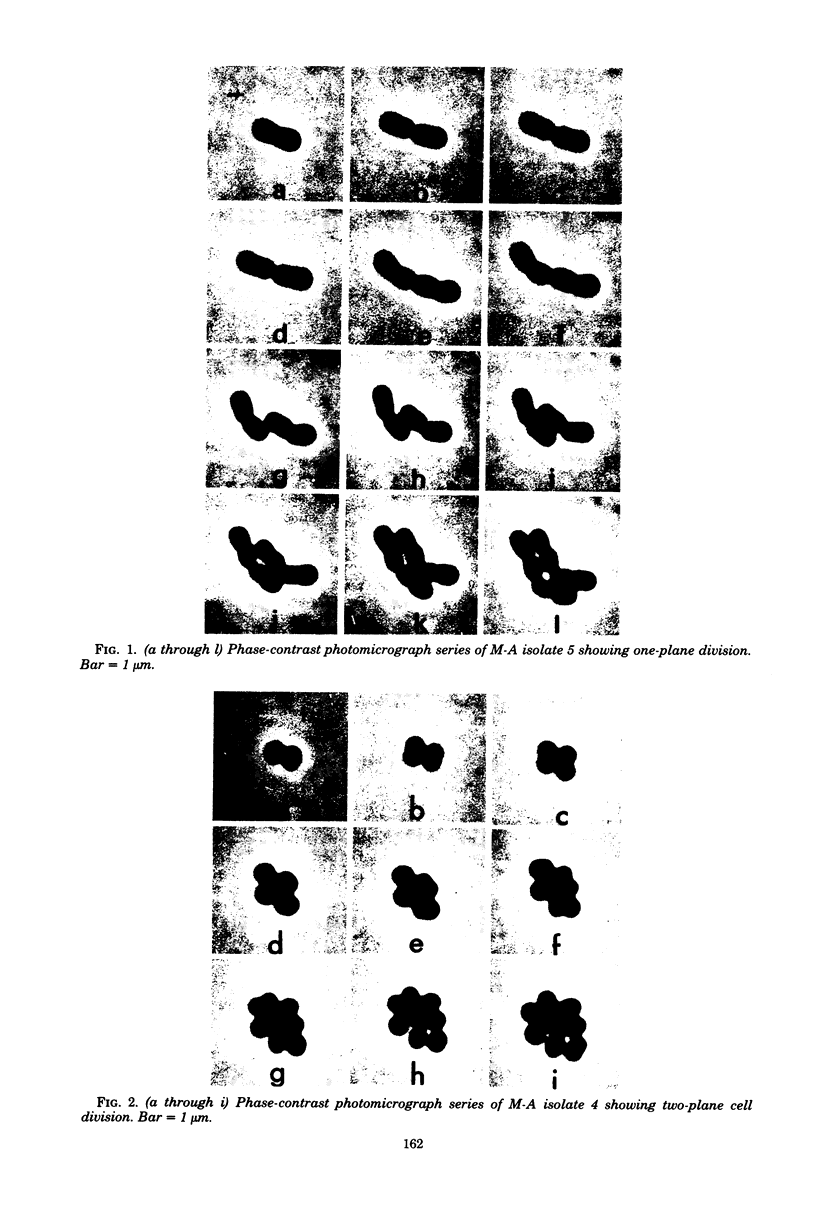
Representative highly radiation-resistant Moraxella-Acinetobacter (M-A), Pseudomonas radiora, Micrococcus radiodurans, and Micrococcus radiophilus exhibited a wide variety of division systems and cell wall characteristics. However, the most resistant M-A possessed unusually thick cell walls, indicating a possible role of the cell wall in radiation resistance in the M-A. Thick septation was present in most of the bacteria studied, but was absent in P. radiora, thus excluding this as a necessity for high resistance. Reliable determination of the number of division planes of the M-A for use as a taxonomic criterion was achieved by the direct observation of dividing cells. The highly resistant M-A were found to divide in multiple planes and had base compositions of 54.0 to 57.5%, unlike typical Moraxella and/or Acinetobacter species. The taxonomic position of most highly resistant bacteria remains unclear.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIRD-PARKER A. C. THE CLASSIFICATION OF STAPHYLOCOCCI AND MICROCOCCI FROM WORLD-WIDE SOURCES. J Gen Microbiol. 1965 Mar;38:363–387. doi: 10.1099/00221287-38-3-363. [DOI] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. The resistance of Micrococcus radiodurans to ultraviolet radiation. 3. A repair mechanism. Biochim Biophys Acta. 1966 Jul 20;123(1):26–33. doi: 10.1016/0005-2787(66)90155-9. [DOI] [PubMed] [Google Scholar]
- De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. Fine structure and radiation resistance in Acinetobacter: a comparison of a range of strains. J Cell Sci. 1971 Jan;8(1):19–41. doi: 10.1242/jcs.8.1.19. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- HUTCHINSON F., ARENA J. Destruction of the activity of deoxyribonucleic acid in irradiated cells. Radiat Res. 1960 Jul;13:137–147. [PubMed] [Google Scholar]
- HUTCHINSON F. The distance that a radical formed by ionizing radiation can diffuse in a yeast cell. Radiat Res. 1957 Nov;7(5):473–483. [PubMed] [Google Scholar]
- KAPLAN H. S., ZAVARINE R. Correlation of bacterial radiosensitivity and DNA base composition. Biochem Biophys Res Commun. 1962 Aug 31;8:432–436. doi: 10.1016/0006-291x(62)90291-7. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Cell wall and cytoplasmic membrane of Escherichia coli. J Biophys Biochem Cytol. 1958 May 25;4(3):323–326. doi: 10.1083/jcb.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobatake M., Kurata H., Komagata K. A radioresistant Gram-positive asporogenous rod isolated from the faeces of a giant panda (Ailuropoda melanoleuca). J Gen Microbiol. 1977 May;100(1):43–48. doi: 10.1099/00221287-100-1-43. [DOI] [PubMed] [Google Scholar]
- Kobatake M., Tanabe S., Hasegawa S. Nouveau Micrococcus radiorésistant à pigment rouge, isolé de fèces de Lama glama, et son utilisation comme indicateur microbiologique de la radiostérilisation. C R Seances Soc Biol Fil. 1973;167(10):1506–1510. [PubMed] [Google Scholar]
- Lavin M. F., Jenkins A., Kidson C. Repair of ultraviolet light-induced damage in Micrococcus radiophilus, an extremely resistant microorganism. J Bacteriol. 1976 May;126(2):587–592. doi: 10.1128/jb.126.2.587-592.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N. F. Studies on a radio-resistant coccus isolated from Bombay duck (Harpodon nehereus). J Gen Microbiol. 1971 Apr;66(1):29–35. doi: 10.1099/00221287-66-1-29. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Mandel M., Igambi L., Bergendahl J., Dodson M. L., Jr, Scheltgen E. Correlation of melting temperature and cesium chloride buoyant density of bacterial deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):333–338. doi: 10.1128/jb.101.2.333-338.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel R. E. Ionizing radiation damage in Micrococcus radiodurans cell wall: release of polysaccharide. Radiat Res. 1976 Apr;66(1):158–169. [PubMed] [Google Scholar]
- Moseley B. E., Copland H. J. Involvement of a recombination repair function in disciplined cell division of Micrococcus radiodurans. J Gen Microbiol. 1975 Feb;86(2):343–357. doi: 10.1099/00221287-86-2-343. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Copland H. J. The rate of recombination repair and its relationship to the radiation-induced delay in DNA synthesis in Micrococcus radiodurans. J Gen Microbiol. 1976 Apr;93(2):251–258. doi: 10.1099/00221287-93-2-251. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Mattingly A., Copland H. J. Sensitization to radiation by loss of recombination ability in a temperature-sensitive DNA mutant of Micrococcus radiodurans held at its restrictive temperature. J Gen Microbiol. 1972 Sep;72(2):329–338. doi: 10.1099/00221287-72-2-329. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Kocur M., Glauert A. M., Thornley M. J. A study by freeze-etching of the fine structure of Micrococcus radiodurans. Arch Mikrobiol. 1973 Dec 4;94(1):77–87. doi: 10.1007/BF00414079. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Silva M. T., Kocur M., Lewis N. F. The fine structure of Micrococcus radiophilus and Micrococcus radioproteolyticus. Arch Microbiol. 1976 Apr 1;107(3):313–320. doi: 10.1007/BF00425346. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M. Fine structure and radiation resistance in Acinetobacter: studies on a resistant strain. J Cell Sci. 1968 Jun;3(2):273–294. doi: 10.1242/jcs.3.2.273. [DOI] [PubMed] [Google Scholar]
- Tzagoloff H., Novick R. Geometry of cell division in Staphylococcus aureus. J Bacteriol. 1977 Jan;129(1):343–350. doi: 10.1128/jb.129.1.343-350.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORK E. AMINO ACIDS OF WALLS OF MICROCOCCUS RADIODURANS. Nature. 1964 Mar 14;201:1107–1109. doi: 10.1038/2011107a0. [DOI] [PubMed] [Google Scholar]
- Welch A. B., Maxcy R. B. Characterization of radiation-resistant vegetative bacteria in beef. Appl Microbiol. 1975 Aug;30(2):242–250. doi: 10.1128/am.30.2.242-250.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work E., Griffiths H. Morphology and chemistry of cell walls of Micrococcus radiodurans. J Bacteriol. 1968 Feb;95(2):641–657. doi: 10.1128/jb.95.2.641-657.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]