Abstract
Dendritic cells (DC) are increasingly exploited for cell-based immunotherapy. However, limitations in ex vivo DC growth and DC functional heterogeneity have motivated development of complementary antigen-presenting cell sources. Here, the ability of CD40 ligand (CD40L)-activated B cells to fulfil that role was investigated. We demonstrate for the first time that non-specific or antigen-specific murine B cells can be grown for extended periods of time by stimulation with CD40L. These cells rapidly up-regulate and maintain high levels of co-stimulatory molecules. In a head-to-head comparison with DC, CD40L-expanded B cells were comparable to DC in the presentation of peptides to CD4+ and CD8+ T cells. While DC were superior to antigen non-specific CD40L-activated B cells with regard to whole protein (NP-BSA) processing and presentation, CD40L-expanded B cells from NP-BSA-immunized mice were comparable to DC when presenting BSA or NP-BSA to primed primary T cells or when presenting NP linked to an unrelated carrier, CGG, to naïve T cells. Thus, the combination of CD40L activation, which supports B-cell growth and augments intracellular protein processing, and antigen uptake via the B-cell receptor, allows for efficient uptake, processing, and presentation of whole protein antigens in a fashion comparable to that observed with mature DC. Like DC, CD40L-activated B cells efficiently home to secondary lymphoid organs in vivo. This system represents a unique tool for studying primary antigen-specific B cells and the results suggest that the outgrowth of large numbers of highly activated B cells represents a viable and practical complement to DC for cell-based immunotherapy.
Keywords: CD40L-activated B cells, dendritic cells, peptide vaccines
Introduction
The recognition that professional antigen-presenting cells (APC) initiate and control immunity to pathogens and malignancies has motivated efforts to design practical, clinically feasible cell-based vaccines. Indeed, since the first description of dendritic cells,1 investigators have appreciated the potential to prepare these APC ex vivo, to load them with tumour lysates, peptides, or genes encoding full-length protein antigens, and to re-inject them to induce immunity. In this regard, numerous preclinical and clinical cancer immunotherapy studies, primarily using dendritic cells (DC) as APC, have been initiated and have shown the safety, feasibility, and efficacy of cell-based vaccination.2–12
Despite these advances, some limitations in the use of DC remain. First, harvesting large numbers of DC needed for multiple rounds of vaccinations, particularly for paediatric patients or patients who have undergone extensive myeloablative chemotherapy, may represent a challenge. A limitation in DC growth potential in vitro precludes long-term expansion of these populations in culture. Second, it is necessary to generate DC at a specific stage of maturation to promote protective immunity rather than T-cell anergy13–15 or regulatory T-cell activation.16,17 Furthermore, even the paradigm that fully mature DC always induce immunity has been challenged.18 In general, the type of DC and the mode of its activation play critical, sometimes complex roles in determining the ability of these APC to effectively induce immunity in vivo.19
Because of these considerations, the use of CD40 ligand (CD40L)-stimulated B cells as alternative or complementary APC to DC has been suggested.20,21 CD40L-activated B cells present antigen to either CD4+ or CD8+ T cells following RNA transfection, retroviral transduction, or direct loading of major histocompatibility complex (MHC) class I with peptides.22–26 Activated B cells offer three potential advantages over DC: (1) B cells are far more frequent in peripheral blood than DC (10–30% versus 0·5–1% of peripheral white blood cells, respectively27,28). (2) Human B-cell populations activated with CD40 ligand and recombinant interleukin-4 (rIL-4) can be expanded for relatively long periods of time in culture, generating hundreds of millions of APC from small blood volumes in a clinically feasible time frame.24,29–32 (3) B cells express antigen-specific surface receptors which can be exploited to capture and augment presentation of tumour- and pathogen-specific antigens.
These putative advantages notwithstanding, the efficacy of activated B cells as APC is seldom compared with that of mature DC in head-to-head analyses.24 Furthermore, the ability of primary CD40L-activated B cells to take up and process whole protein antigens for presentation to naïve CD4+ T cells, a requirement for the induction of robust, long-lasting tumour immunity, has not been well described. To the extent that it has been studied, antigen-non-specific CD40L-activated primary B cells appear to take up and process tumour proteins for MHC class II presentation to CD4+ T cells when the B cells are cultured with relatively large amounts of lysates from tumour cell lines.32 However, the clinical feasibility of this approach is uncertain given finite amounts of primary tumour available to generate lysates and the minimal amount of any given protein antigen within the lysate.
A more efficient approach would be to exploit the B-cell receptor (BCR) to maximize internalization and processing of protein antigens for presentation by primary B cells to CD4+ T cells. Indeed, antigen processing and presentation to T-cell hybridomas by antigen-specific B cells is 1000–10 000 times more efficient than that of non-specific B cells.33–35 Furthermore, CD40L activation of antigen non-specific B cells increases their ability to process and present protein antigens to immortalized T cells.36 Here we combined several of these characteristics of activated B cells, i.e. the ability to expand primary B-cell populations in vitro, enhanced antigen processing by B cells following CD40L signalling, and increased antigen uptake through the BCR, to demonstrate the feasibility of preparing large numbers of efficient, antigen-specific primary B cells for antigen presentation to naïve or primed primary T cells. In so doing, we present the results of a direct head-to-head comparison between CD40L-activated B cells and mature DC with regard to presentation of MHC class I- and II-restricted peptides and whole protein antigens. Furthermore, we address the possibility of inducing hapten-specific B cells in vivo, activating and expanding them in vitro with CD40L, and exploiting their hapten-specific BCR to facilitate uptake, processing, and presentation of unrelated protein antigens conjugated with the immunizing hapten to naïve primary T cells.
Materials and methods
Mice
C57BL/6J (H-2b), C57BL/6-Tg(HLA-A2.1)1Enge/J, and C57BL/6.GFP mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in the AAALAC-accredited Boston University Laboratory Animal Science Center and used at 5–8 weeks of age. These studies were reviewed and approved by the Boston University Institutional Animal Care and Use Committee, protocol # AN-14052.
Antigens
Nitrophenol-conjugated bovine serum albumin (NP-BSA) and chicken γ-globulin (NP-CGG) were synthesized by Bioresearch Technology (Novarto, CA). BSA was purchased from Sigma-Aldrich Co. (St. Louis, MO). Peptides were synthesized by Dr C. Dahl (Harvard Medical School, Boston, MA). Peptide sequences are as follows: myelin oligodendrocyte glycoprotein (MOG) 35–55: MEVGWYRSPFSRVVHLYRNGK; myelin basic protein (MBP) 59–76: HTRTTHYGSLPQKSQHGR.
Activation and growth of B cells with CD40L
Spleen cells were stimulated by growth on monolayers of irradiated, human CD40L-tranfected L cells. Expression of CD40L was regularly tested by flow cytometry using fluorescein isothiocyanate (FITC)-conjugated human CD40L-specific antibody (BD Biosciences, San Jose, CA) and cells were periodically sorted for high CD40L expression on a Dako MoFlo cell sorter (Dako, Fort Collins, CO). CD40L cells were irradiated (3000 rad) and plated in six-well plates (Corning, Corning, NY) at a concentration of 0·4 × 105 cells per well in Dulbecco's modified Eagle's minimal essential medium supplemented with 10% fetal calf serum (FCS), 1% HEPES, 1%l-glutamine, and 1% penicillin/streptomycin (Sigma-Aldrich). Adherent CD40L-expressing L cell monolayers were established within 12–14 hr. Murine splenocytes (1·25 × 106 cells/ml) were added to the monolayers and the cultures were supplemented with 10 ng/ml rIL-4 (RDI/Fitzgerald Industries Intl, Concord, MA). To prevent outgrowth of T cells, cyclosporin A (5·5 × 10−7 m; Sigma–Aldrich) was added. Cells were transferred to fresh monolayers of irradiated CD40L cells every 3–4 days, with cyclosporin A present only for the first two passages. Cell number and viability were established by trypan blue exclusion. The percentage of B220+ cells was determined by flow cytometry using FITC- or phycoerythrin (PE)-conjugated B220-specific antibody (BD Biosciences). After two passages, cultures consisted of >95% B220+ B cells.
Mature dendritic cells
Dendritic cells were generated from bone marrow as previously described.37 Briefly, bone marrow was flushed from mouse femurs, dissociated, washed, and cultured at an initial density of 2 × 105 cells/ml in RPMI-1640 supplemented with 2 mm l-glutamine, 50 μm 2-mercaptoethanol, 10% FCS, 60 U/ml recombinant granulocyte–macrophage colony-stimulating factor (rGM-CSF) and 100 U/ml rIL-4. The medium was replenished at day 2 and cells cultured for an additional 4–5 days. Maturation was induced by adding 50 ng/ml tumour necrosis factor-α (TNF-α; RDI/Fitzgerald Industries). Mature DC were washed and harvested by gentle scraping 3 days later.
Flow cytometry
Naïve B cells, CD40L-activated B cells, or mature DC (106) were incubated with 50 μl of Fc block (BD Biosciences) for 30 min. Cells then were incubated for 30 min on ice with FITC-labelled antibody specific for MHC class I (H-2Kb), MHC class II (I-Ab/I-Eb), CD80, or CD86 and PE-conjugated antibody specific for B220 or CD11c (BD Biosciences). Cells were washed and fixed in 1% paraformaldehyde before analysis in a Becton-Dickinson FACScan flow cytometer (Becton Dickinson, San Jose, CA).
To quantify the percentage of NP-BSA-specific B cells after immunization, spleens were excised, dissociated, and white blood cells enriched on lymphocyte separation media (MP Biomedicals, Solon, OH). Cells (106) then were incubated with 50 μl of 1 mg/ml CGG for 30 min. followed by incubation with 1 mg/ml FITC–NP-BSA (Bioresearch Technology, Novarto, CA) for 1 hr. Cells were washed and treated with 50 μl Fc block for 30 min on ice. Cells then were washed and co-stained with PE–anti-B220 antibody, then fixed in 1% paraformaldehyde before analysis. CD40L-activated B cells were similarly stained with FITC–NP-BSA 4 days after initiation of culture with CD40L-transfected L cells and rIL-4. Staining of cells cultured for longer than 4 days was precluded by an increase in the non-specific binding of FITC–NP-BSA by B-cell populations expanded from non-immunized donors.
To determine the percentage of CD40L-activated B cells homing to spleen and lymph nodes, 106 splenic or lymph node cells from mice injected intravenously (i.v.) 7 or 10 days previously with CD40L-activated B cells were incubated with 50 μl of Fc block (BD Biosciences) for 30 min. Cells then were incubated for 30 min on ice with PE-isotype control or PE–anti-B220 antibody. Cells were washed, fixed in 1% paraformaldehyde, and analysed by flow cytometry. Quadrants were set using PE-isotype controls and splenic cells from wild type C57BL/6 mice.
T-cell responses
T-cell clones were kindly provided by Dr. Sara Abromson-Leeman (Harvard Medical School, Boston, MA). The MBP59–76-specific CD4+ clone (‘Clone 12’) was generated in a C57BL/6 interferon-γ receptor null mouse and was maintained with peptide-pulsed irradiated (3000 rad) C57BL/6 spleen cells. The MOG35–55-specific CD8+ clone 7 was generated in C57BL/6-Tg(HLA-A2.1)1Enge/J transgenic mice and was maintained with peptide-pulsed irradiated C57BL/6-Tg(HLA-A2.1)1Enge/J spleen cells. APC were pulsed with 10 μg/ml peptide for 2 hr at 37° with 3 μg/ml β2-microglobulin in phosphate-buffered saline (PBS), washed extensively, and irradiated (3000 rad). MBP- and MOG-specific clones were co-cultured for 36 hr in triplicate wells at a density of 5 × 103 cells/well in 96-well plates with 2·5 × 103 or 1·25 × 103 peptide-pulsed CD40L-activated B cells or mature DC. 3H-thymidine (1 μCi/well) was added and thymidine incorporation assayed 18–20 hr thereafter. Results from triplicate wells were averaged for each experiment.
Immunization
C57BL/6 mice were immunized intraperitoneally (i.p.) three times at 14 day intervals with 125 μg NP-BSA or NP-CGG emulsified in 0·5 ml complete Freund's adjuvant (CFA; Sigma-Aldrich). Controls were injected with 0·5 ml of a PBS/CFA emulsion. Mice were killed 10 days after the last immunization, spleens harvested, and splenocytes used to generate CD40L-activated B cells or to purify primary responder T cells. Bone marrow of PBS/CFA-immunized mice also was used to generate DC to directly compare CD40L-activated B cells and DC from mice injected with a CFA/PBS emulsion.
Presentation of whole protein antigen by CD40L-activated B cells and dendritic cells
CD40L-activated B cells derived from PBS/CFA-treated or NP-BSA-immunized C57BL/6 mice and DC from CFA/PBS-treated mice were pulsed with 1 μg/ml NP-BSA, BSA, or NP-CGG for 12 hr in serum-free medium with rIL-4. (Preliminary experiments indicated a loss of antigen-presenting capacity when cells were pulsed with <0·5 μg/ml antigen.) Cells were harvested, washed extensively, and irradiated (3000 rad). Responder T cells were isolated from spleens of NP-BSA/CFA-immunized or PBS/CFA-treated C57BL/6J mice using the RosetteSep Kit (Stem Cell Technology, British Columbia, CA) and following the manufacturer's instructions. T cells (105; ≥90% CD3+) and irradiated, protein-pulsed APC (5 × 104) were co-cultured in triplicate wells of a 96-well plate for 30 hr. 3H-thymidine was added and thymidine incorporation assayed 18 hr thereafter.
Statistical analyses
Statistical analyses were performed with Statview (SAS Institute, Cary, NC). Data from a minimum of three independent APC cultures (triplicates for each culture were averaged) are presented as means ± standard errors (SE). The Student's t-test and one-factor anovas were used to analyse the data. For the anova, Scheffe's multiple comparisons test was used to determine significant differences.
Results
Expansion of primary murine B-cell populations
It has been suggested that CD40L-activated B cells may serve as effective tumour APC in the clinical setting.29,38 Indeed, limitations in growing large numbers of DC for repeated human immunizations, and the relative ease with which human B-cell populations can be expanded with CD40L activation, suggest that CD40L-activated B cells may complement DC for cell-based vaccination.20 However, models systems in which this hypothesis can be tested have been limited by the difficulty in expanding primary murine B-cell populations as easily as human B-cell populations. Therefore, our initial experiments were directed towards maximizing the stimulus provided by CD40L such that large numbers of activated murine B cells could be generated to compare their ability to present antigen with that of mature DC.
Experiments using a fusion protein consisting of murine CD40L linked to a CD8 epitope and CD8-specific antibodies to cross-link the fusion protein39 succeeded in inducing murine B cells to blast and, to a limited extent, to divide (data not shown). However, these B cell populations could not be expanded in vitro for significant lengths of time, i.e. more than 5 days.
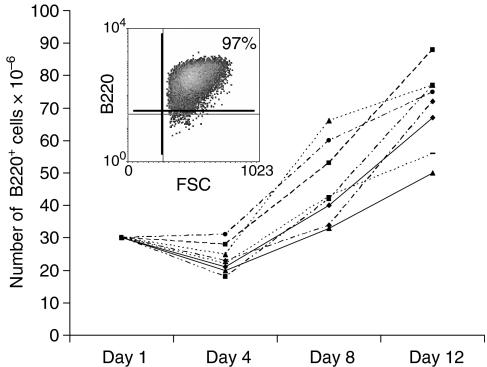
As an alternative approach, human CD40L-transfected L cells were stained with FITC-labelled CD40L-specific antibody, sorted for the brightest 10%, irradiated (3000 rad), and maintained in monolayers to stimulate murine B cells. Indeed, stimulation of whole murine splenocytes with CD40Lhigh L cells in the presence of rIL-4 to increase cell viability, and in the presence of cyclosporin A to eliminate T cells, resulted in the rapid outgrowth and expansion of highly enriched, large blasting B cells that were >95% B220+ by day 7 (Fig. 1, insert). In eight consecutive cultures, approximately 30 × 106 splenocytes (∼15–20 × 106 B220+ B cells) generated 70 ± 5 × 106 B220+ B cells within the first 12 days of culture (Fig. 1). Most cultures of murine B cells could be maintained for several weeks thereafter. Removal of the B cells from CD40L-transfected L cell monolayers resulted in B cell death within 4 days. Similar results were obtained with human B cells (not shown), although human B cells could be continually expanded for significantly longer periods of time (>8 weeks).
Figure 1.
Murine B-cell populations can be expanded by stimulation with CD40L. Spleen cell populations were phenotyped for B220 expression and cultured in duplicate wells for 12 days on CD40L-transfected L cells in the presence of rIL-4. Duplicate wells were harvested on days 4, 8, and 12 and the number of viable (trypan blue excluding), B220+ B cells determined. Data represent the total number of B220+ B cells recovered at various time points in 8 independent cultures. A representative B220 phenotype of cells after 7 days of culture is presented in the insert.
From these results it is concluded that these culture conditions are sufficient for modeling production of human CD40L-activated B cells for cell vaccination. Furthermore, these results suggest, for the first time, a system for evaluating the biological function of non-transformed, non-transgenic murine B cells that can be sustained in culture for relatively long periods of time. As shown below, these studies can include the analysis of antigen-specific B-cell function.
CD40L-activated murine B-cell populations and mature dendritic cells express comparable levels of co-stimulatory molecules
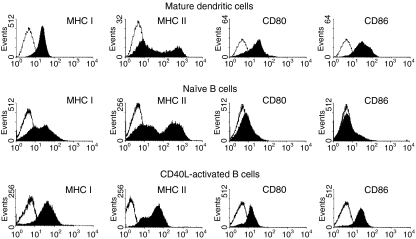
Because sufficient expression of MHC antigens and costimulatory molecules closely correlates with APC function,40,41 a phenotypic analysis of cell surface markers on CD40L-activated B cells and on bone marrow-derived mature DC was undertaken. Bone marrow DC expanded for 7 days with 60 U/ml rGM-CSF and 100 U/ml rIL-4 and matured for 3 days with 50 ng/ml rTNF-α expressed significant levels of MHC class I, MHC class II, CD80, and CD86 (Fig. 2, top row). While subpopulations of non-stimulated, naïve, murine B cells expressed MHC class I and MHC class II antigens, they did not express detectable levels of CD80 or CD86 (Fig. 2, middle row). However, growth of B cells on CD40L-transfected L cells significantly up-regulated MHC class I and class II expression and induced expression of CD80 and CD86 to levels similar to those detected on mature DCs (Fig. 2, bottom row). The B cells maintained this phenotype throughout the culture period. These results are consistent with those obtained with human B cells31 and further suggest that CD40L-activated B cells may be highly competent APC.
Figure 2.
Murine CD40L-activated B cells exhibit a similar co-regulatory molecule phenotype as dendritic cells. Mature DC were generated from murine bone marrow by culture with rIL-4 and rGM-CSF for 4 days followed by a two day culture with rTNF-α. CD40L-activated B cells were generated by culture for 12 days on monolayers of L cells transfected with human CD40L in the presence of rIL-4 and cyclosporin A. Cells were phenotyped using a combination of B220-specific antibody and antibody specific for MHC I, MHC II, CD80, or CD86. Co-regulatory molecule expression (solid histograms) on cells gated for B220 expression is presented. Open histograms represent data generated with FITC-isotype control antibodies. Data from a representative experiment (>5 total) are presented.
CD40L-activated murine B-cell populations and mature dendritic cells are comparable with respect to self peptide presentation to CD4+ and CD8+ T-cell clones
The comparable levels of MHC class I and II molecules and of costimulatory molecules on mature DC and CD40L-activated B cells suggest that these two types of APC may be similar in their ability to present MHC-binding peptide antigens that do not require antigen uptake or intracellular processing. To test this hypothesis, CD40L-activated murine B cells, expanded for a minimum of 12 days, and DC were pulsed with MHC class I- or MHC class II-binding peptides, and the responses of peptide/MHC-restricted CD4+ and CD8+ T cell clones assayed by 3H-thymidine incorporation. The peptides chosen for these experiments were derived from autoantigens, i.e. MBP and MOG, as it is anticipated that at least one application of CD40L-activated B cells will be to present self (i.e. tumour)-associated antigens.
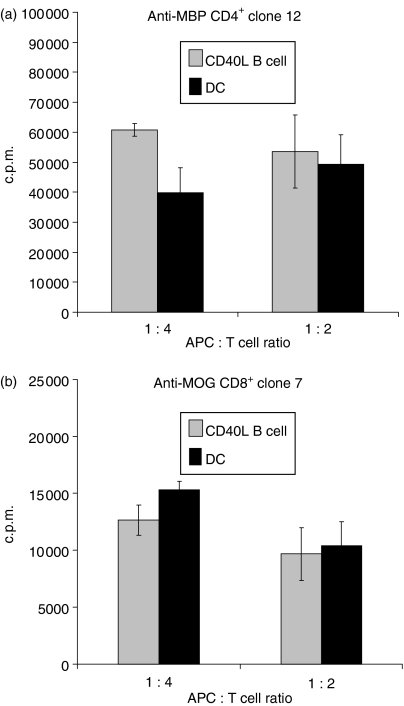
At APC to responder T-cell ratios of 1 : 4 and 1 : 2, CD40L-activated B cells were at least as efficient as DC at presenting the MHC class II-binding self peptide, MBP56–76, to CD4+ T-cell clone 12 (Fig. 3a). Similar data were obtained with a second MOG peptide-specific CD4+ T-cell clone (not shown). Similarly, CD40L-activated B cells were comparable to DC when presenting the MHC class I-binding self peptide, MOG35–55, to CD8+ T-cell clone 7 (Fig. 3b). These data indicate that primary CD40L-expanded B cells are efficient presenters of self peptides to both CD4+ and CD8+ T cells.
Figure 3.
Murine CD40L-activated B cells are comparable to mature dendritic cells (DC) in peptide presentation to CD4+ and CD8+ T-cell clones. Bone marrow DC or splenic CD40L-activated and expanded B-cell populations were generated as in Fig. 1 and loaded with 10 μg/ml MOG or MBP peptides, irradiated and cultured for 36 hr with MBP/MHC II-restricted CD4+ T cells (‘clone 12’) or MOG/MHC I-restricted, CD8+ T cells (‘clone 7’). 3H-thymidine was added and CPM incorporation determined 18–20 hr later. Data obtained from 3 independent cultures of APC are presented as means (3H-thymidine incorporation in the presence of peptide – 3H-thymidine incorporation in the absence of peptide) ± SE. Background incorporation of CD8+ and CD4+ T-cell clones cultured in the presence of APC but without peptide averaged <600 c.p.m. and <2000 c.p.m., respectively.
Antigen-specific CD40L-activated B cells efficiently endocytose and present protein antigen to primed primary T cells
Dendritic cells activated through innate stimuli efficiently endocytose and process whole proteins for peptide presentation to both CD4+ and CD8+ T cells.42 While CD40L-activated B cells may present sufficient levels of peptide antigens after culture with relatively high doses of protein,32 they likely are less efficient at endocytosing whole proteins than DC. However, antigen-specific BCRs may be exploited to optimize uptake of whole protein antigens. Therefore, studies were designed that directly compared the ability of primary antigen-specific B-cell populations, expanded for extended periods of time with CD40L, and DC to process and present whole proteins.
C57BL/6 mice were immunized i.p. with NP-BSA emulsified in CFA three times over a 6-week period. Control mice were injected i.p. with CFA emulsified with PBS alone (PBS/CFA). Mice were killed 10 days after the final immunization, spleens removed, and splenocytes co-stained with FITC–NP-BSA and PE–anti-B220 antibody to determine the percentage of NP-BSA-specific B cells.
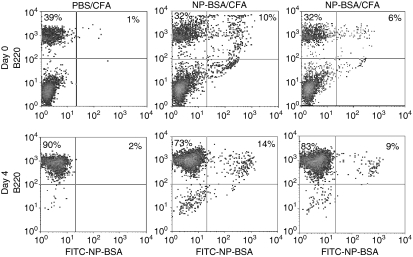
A small fraction (≤1%) of lymphocytes, as defined by forward and side scatter parameters, from spleens of control PBS/CFA-treated mice stained non-specifically with FITC–NP-BSA (Fig. 4, upper left dot plot). In contrast, 8·3 ± 2·0% (nine mice) of the lymphocytes from NP-BSA-immunized mice stained with FITC–NP-BSA (Fig. 4, center and upper right dot plot). The percentage of hapten/carrier (NP-BSA)-specific B cells observed after three immunizations of NP-BSA in CFA is comparable to that seen after two immunizations with NP-keyhole limpet haemocyanin in RIBI adjuvant (∼10% of spleen; RIBI ImmunoChem Research, Inc, Hamilton, MT).43 Culture of these cells on monolayers of CD40L-transfected L cells resulted in outgrowth of B cells, 10·5 + 6·7% (cultures from six mice) of which bound FITC–NP-BSA (Fig. 4, lower right dot plot). A drop in the percentage of antigen-specific B cells, as a percentage of all B cells, was noted after initiation of culture. However, this decrease appeared to be transient and related only to adaptation of cells to culture. That is, the percentage of B cells that bound NP-BSA stabilized over the first few days of culture (not shown).
Figure 4.
Growth of antigen-specific B cells after CD40L activation. Mice were injected three times with PBS/CFA or NP-BSA/CFA. Ten days after the last treatment, mice were killed and an aliquot of spleen cells stained with PE–anti-B220 antibody and with FITC–conjugated NP-BSA. The remainder of the spleen cells were cultured on CD40L-transfected L cells in the presence of rIL-4 and cyclosporin A for 4 days and phenotyped. Representative data from a total of 9 mice on day 0 and 6 mice on day 4 are presented.
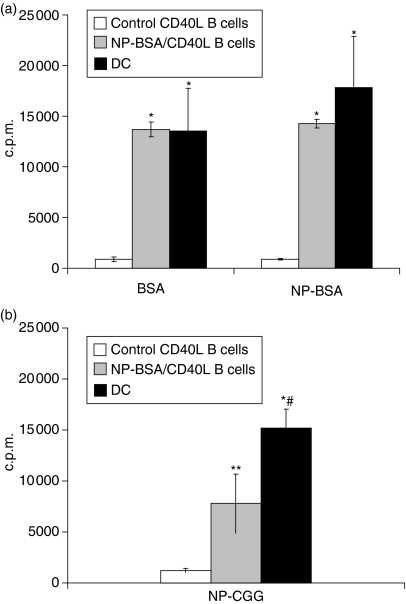
To determine how DC and CD40L-activated non-specific B cells compare with these CD40L-activated antigen-specific B cells with regard to protein uptake, processing, and presentation, CD40L-expanded B cells and DC from PBS/CFA-treated control mice and CD40L-expanded B cells from NP-BSA/CFA-immunized mice were pulsed for 12 hr with 1 μg/ml BSA or NP-BSA. Cells were harvested, extensively washed, irradiated (3000 rad), and co-cultured for 30 hr with purified primed T-cell populations from NP-BSA-immunized mice. 3H-thymidine was added and T-cell proliferation was assayed 18–20 hr later.
CD40L-activated B cells from control mice induced minimal T-cell proliferation to BSA or NP-BSA (Fig. 5a, open bars). In contrast, B cells expanded from NP-BSA-immunized mice (gray bars) and mature DC (black bars) pulsed with 1 μg/ml BSA or NP-BSA induced significant T-cell responses relative to responses induced with CD40L-activated B cells from control mice (P < 0·003). There were no significant differences between responses induced with DC as compared with responses induced with CD40L-activated B cells from NP-BSA-immunized mice. Similarly, both APC lost antigen-presenting function when pulsed with <0·5 μg/ml antigen. This response was highly antigen specific because little or no response was seen when B cells from NP-BSA-immunized mice were pulsed with CGG and used to stimulate T cells from NP-BSA-immunized mice or when B cells from NP-CGG-immunized mice were pulsed with BSA and used to stimulate T cells from NP-CGG-immunized mice (data not shown).
Figure 5.
Antigen-specific CD40L-activated B cells efficiently endocytose and present protein antigen to primed primary T cells. Mice were injected with PBS/CFA or NP-BSA/CFA as in Fig. 4. Ten days after the last treatment, mice were killed and splenocytes cultured for a minimum of 10 days on CD40L-transfected L cells in the presence of rIL-4. CD40L-activated B cells from PBS/CFA-treated (control) mice, CD40L-activated B cells from NP-BSA/CFA-immunized mice, or DC from control mice were pulsed with 1 μg/ml BSA or NP-BSA (a), or NP-CGG (b), irradiated, and 5 × 104 cells added to 105 purified T cells from NP-BSA-immunized mice. After 36 hr, 3H-thymidine was added and c.p.m. incorporation determined 18–20 hr thereafter. Data are presented as means from three independent cultures of each APC (3H-thymidine incorporation in the presence of peptide – 3H-thymidine incorporation in the absence of peptide) ± SE. Background incorporation of T cells cultured in the presence of APC but without peptide averaged <600 c.p.m. and <2800 c.p.m., respectively. Significant differences relative to responses generated in the presence of antigen-pulsed, control CD40L-activated B cells, *P < 0·003 and **P < 0·02, respectively. Significant difference relative to responses generated in the presence NP-CGG-pulsed CD40L-activated B cells from NP-BSA/CFA-immunized mice, #P < 0·03.
To determine if a hapten epitope is sufficient to facilitate whole protein uptake and antigen presentation to T cells, CD40L-activated B cells from NP-BSA-immunized mice and DC from control mice were pulsed with NP-CGG and used as APC for purified T cells from NP-BSA-immunized mice.
As seen following in vitro antigenic challenge with BSA or NP-BSA, NP-CGG-pulsed CD40L-activated B cells from control mice induced minimal T-cell proliferative responses (Fig. 5b, open bar). In contrast, CD40L-expanded NP-CGG-pulsed B cells from NP-BSA immunized mice induced significantly higher proliferative responses (grey bar) than activated B cells from PBS/CFA-treated control mice (P < 0·02), although these responses were lower than those generated with DC (P < 0·03). These results indicate that the presence of a hapten on a protein is sufficient to facilitate BCR-mediated uptake and processing of that antigen by CD40L-expanded B-cell populations. Because the responder T cells were generated from NP-BSA- and not NP-CGG-immunized mice, the results further suggest either that primed T cells recognize an epitope common to NP-BSA and NP-CGG or that antigenically naïve CGG-specific T cells, present in the purified T-cell population, are being induced to respond to CGG-peptide by NP-CGG-pulsed, CD40L-activated B cells.
Antigen-specific CD40L-activated B cells are comparable to dendritic cells with regard to uptake and presentation of protein antigen to naïve primary T cells
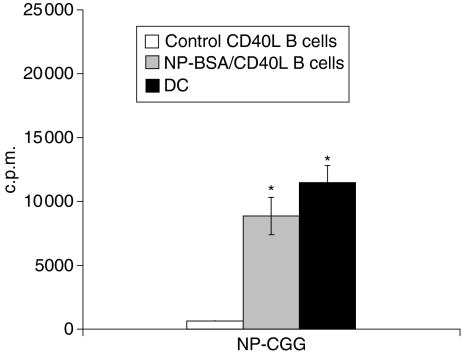
To determine if CD40L-expanded, antigen-specific B cells efficiently endocytose proteins by virtue of a hapten epitope and present peptide antigen(s) to antigenically naïve T cells, and to compare this putative antigen presentation to that exhibited by DC, B cells from control and NP-BSA-immunized mice were expanded for a minimum of 10 days on CD40L-transfected L cells. These activated B cells, and mature DC from PBS/CFA-treated mice, were pulsed with NP-CGG, irradiated, washed, and cultured with T cells from control mice.
Proliferation of responder T cells from control mice was minimal after culture with NP-CGG-pulsed CD40L-expanded B cells from PBS/CFA-treated mice (Fig. 6, open bar). In contrast, significant T-cell proliferation was seen when T cells were challenged with NP-CGG-pulsed, CD40L-expanded B cells from NP-BSA immunized mice (grey bar) or with NP-CGG-pulsed DC (black bar; P < 0·005). The responses were not significantly different than those generated with T cells from NP-BSA-immunized mice after challenge with NP-CGG-pulsed CD40L-B cells from NP-BSA-immunized mice (Fig. 5b), suggesting that most, if not all of the response observed with T cells from NP-BSA-primed mice reflected primary responses of naïve, CGG-specific T cells. These data demonstrate that DC and CD40L-activated, antigen-primed B cells are comparable at presenting neoantigens, as long as the antigen expresses an epitope to facilitate antigen uptake through the epitope-specific BCR.
Figure 6.
Hapten-specific CD40L-activated B cells are comparable to DC in their ability to present hapten–protein to antigen naive, primary T cells. CD40L-activated B cells and DCs were pulsed with 1 μg/ml NP-CGG, washed extensively, irradiated, and cultured for 36 hr with T cells from PBS/CFA-treated mice. 3H-Thymidine was added and c.p.m. incorporation determined 18–20 hr thereafter. Data are presented as means from three independent cultures of each APC (3H-thymidine incorporation in the presence of peptide –3H-thymidine incorporation in the absence of peptide) ± SE. Significant difference relative to responses generated with NP-CGG pulsed CD40L-activated B cells expanded from control mice, *P < 0·005.
CD40L-activated B cells migrate to secondary lymphoid organs
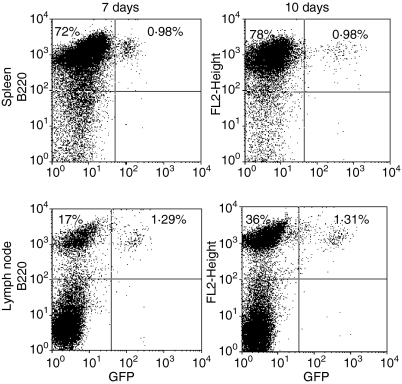
In order for CD40L-activated B cells to serve as useful APC in vivo, it is necessary that they have the capacity to home to secondary lymphoid organs. Previous studies with human CD40L-activated B cells that characterized their expression of surface homing molecules, strongly suggested that CD40L-activated B cells can home to secondary lymphoid organs.44 To test this hypothesis, B cells from C57BL6.GFP transgenic mice were activated by culture on CD40L-transfected L cells with rIL-4 for 10 days and injected i.v. into C57BL/6 mice. Inguinal lymph nodes and spleens were harvested 7 and 10 days later and evaluated for the presence of B220+/GFP+ B cells.
A significant number of B220+/GFP+ were detected in both the inguinal lymph nodes and spleens of B cell-injected mice (Fig. 7). The average percentages of B220+ cells expressing GFP in spleen and lymph nodes 7 days after injection were 1·9% and 1·4%, respectively and 10 days after injection were 1·4% and 2·0%, respectively. These results suggest that CD40L-activated B cells efficiently home to lymphoid organs where, presumably, they are capable of presenting antigen to resident T cells.
Figure 7.
CD40L-activated B cells efficiently home to secondary lymphoid organs. Single-cell suspensions from GFP transgenic mice were cultured on CD40L-transfected L cells in the presence of rIL-4 and cyclosporin as in the legend to Fig. 1. Seven days later cells were washed and 3 × 107 cells injected i.v. into C57BL/6 mice (four mice/group). Mice were killed 7 or 10 days later. Spleens and inguinal lymph nodes were removed, stained with PE-labelled B220-specific or isotype-control antibody and analysed by flow cytometry. Quadrants were set using PE–isotype antibody-treated C57BL/6 spleen or lymph node cells. Data presented were gated on lymphocytes, as determined by forward and side scatter parameters, and are representative of results obtained in each of four mice killed 7 and 10 days after cell injection. The percentage of cells falling into the B220+/GFP− and B220+/GFP+ upper left and upper right quadrants respectively are indicated.
Discussion
DC are critical for linking innate and adaptive immune responses in vivo. They are clearly the most potent APC when protein antigens are first introduced to the immune system concomitant with innate ‘danger’ signals. They also are the most efficient APC at antigen cross-presentation via the MHC class I pathway.45 Furthermore, their ability to initiate adaptive immune responses in the clinical setting has been demonstrated.46,47 Nevertheless, the identification of an increasing number of DC subsets,19,48 including those which induce suppressive T regulatory cells,17,18 suggests that great care must be taken to generate the appropriate DC subset at the proper stage of differentiation prior to vaccination.
Furthermore, the use of DC for repeated immunizations may be somewhat limited by the difficulty in significantly expanding DC populations in vitro. For example, the highest dose of RNA-transfected DC used for immunotherapy for prostate and renal cell carcinoma patients was set at 0·5 × 108 cells/vaccination for each of three vaccinations, because approximately 1·5 × 108 was the maximum number of immature DC obtainable after patient leukapheresis.46,47 Multiple vaccinations with high numbers of APC appears justified given animal studies demonstrating a steep drop-off of T-cell activation with twofold decreases in the number of ovalbumin-pulsed DC.49 Similarly, a protocol designed specifically to maximize the number of DC obtained from adult leukapheresis product generated a maximum of 1·5 × 109 DC.50 The generation of sufficient numbers of DC may be even more problematic in paediatric patients.20,24 In contrast, 109 CD40L-activated B cells can be readily generated from approximately 10 ml of blood.20 Orders of magnitude more fresh CD40L-expanded B cells can be generated from leukapheresis product. Thus, consideration of CD40L-activated and expanded B cells as a complementary source of APC is warranted.
Because clinical trials with CD40L-activated B cells have begun, it seems prudent to compare the efficacy of these cells to that of DC, and to determine if the BCR can be exploited to increase antigen uptake and thereby antigen presentation to both primed and unprimed T cells. To accomplish this, it was first necessary to establish conditions in which outgrowth of murine B cells would model conditions for expansion of CD40L-activated human B cells in a clinical setting. As presented herein, outgrowth of murine B cells was accomplished by culture of splenocytes on monolayers of fibroblasts expressing high levels of human CD40L. The failure to maintain murine B cells in culture for significant lengths of time when using a soluble form of a CD40L–CD8 fusion protein cross-linked with anti-CD8-specific antibodies39 suggests that transfected fibroblasts provide greater opportunity for multivalent CD40L signalling. Indeed, this culture system allows the growth of primary murine B-cell populations for several weeks, an outcome long sought by B-cell immunologists. Although CD40L-activated human B cells can be maintained for somewhat longer periods of time than murine cells, the murine B-cell cultures were sufficient to evaluate up-regulation of co-stimulatory molecules, growth of non-specific B-cell populations, and antigen presentation by expanded antigen-specific B-cell populations. As such, this model significantly facilitates evaluation of the biological function of non-transformed, non-transgenic murine B cells.
As shown for CD40L-activated human B cells, activated murine B cells rapidly up-regulate and maintain expression of both MHC and co-stimulatory molecules required for antigen presentation. The levels of these molecules are comparable to those observed on mature dendritic cells. As would be predicted from this result, and consistent with studies performed with human CD40L-activated B cells,25,26,31 CD40L-expanded murine B-cell populations induced significant proliferative responses to both CD4+ and CD8+ T-cell clones when pulsed with MHC class II- and MHC class I-binding peptides respectively. These outcomes also strongly suggest that CD40L-activated B cells will be effective in inducing CTL responses as well as cytokine-mediated T helper activity. The result that comparable responses of T cells were obtained with CD40L-expanded B cells and mature DC, even at lower APC : T-cell ratios, suggests that the former cell type is likely to be as effective at inducing clinically meaningful responses to peptide antigens on a per cell basis as the latter.
Pulsing of peptides directly onto MHC class I or II on CD40L-activated B cells requires little of the antigen-presenting B cell other than expression of MHC antigens and co-stimulatory molecules. Although a powerful approach to cellular vaccination, peptide pulsing does not address the capacity of CD40L-expanded populations to process and present protein antigens. The generation of robust CD8+ T-cell responses with RNA-transfected CD40L-activated human B cells24 demonstrates that CD40L-activated B cells can at least process endogenously encoded proteins through the MHC class I pathway. However, their failure to generate significant T-cell responses when incubated with modest concentrations of protein antigen (e.g. Fig. 5, open bars) indicates that uptake of whole proteins by CD40L-activated B cells is limited. Unless relatively large concentrations of antigen are available,32 it seems unlikely that pulsing of CD40L-activated B cells with whole protein will represent a practical approach to cellular immunotherapy.
This limitation notwithstanding, it has been suggested that the BCR can be exploited to enhance antigen uptake and processing33,36,51–53 potentially through human leucocyte antigen-DO-mediated inhibition of MHC class II loading of endogenous B-cell peptides.54 Most of these studies used antigen-coupled immunoglobulin-specific antibodies, non-activated B cells from immunoglobulin transgenic mice, or immortalized T cells to demonstrate antigen presentation by antigen-specific B cells. None of the studies compared antigen presentation by B cells to that provided by DC. In addition, it has been shown that CD40L activation enhances B-cell processing of protein antigens.36 Consequently, we chose to combine both aspects of B-cell antigen presentation to optimize antigen uptake, processing, and presentation by B-cell populations expanded in vitro and to directly compare that activity to the activity of antigen non-specific DC. Indeed, B-cell populations from NP-BSA-immunized mice, expanded in vitro for a minimum of 10 days, efficiently presented either NP-BSA or BSA to primed T cells. On a per cell basis, activated B cells from immunized mice were as effective as DC at presenting either antigen. Indeed, the efficiency with which antigen-specific CD40L-activated B cells may induce responses may be underestimated in these studies because only a fraction (10·6 ± 1·7%) of the B-cell populations were capable of binding NP-BSA.
Because hapten-specific B cells can be generated by immunization with hapten–protein conjugates, it was possible to determine if BCR specificity for hapten alone could be exploited to facilitate neoantigen uptake and processing. The induction of robust immune responses to NP-CGG with CD40L-expanded B-cell populations from NP-BSA-immunized mice (Fig. 6) supports this hypothesis and suggests the possibility of enhancing antigen presentation, in vitro or in vivo, of a relatively non-immunogenic, hapten-coupled protein after conventional immunization with hapten conjugated to an immunogenic protein (e.g. tetanus toxoid). Additional experimentation would be required to demonstrate that the T-cell responses observed here after in vitro challenge with NP-CGG were not exclusively hapten specific, although hapten-specific T-cell responses are historically generated with hapten-modified APC, not with hapten–protein conjugates.55,56
In any case, the ability of expanded B-cell populations from NP-BSA-immunized mice to present NP-CGG to antigenically naïve T cells (Fig. 6) demonstrates for the first time that B-cell populations activated and expanded in vitro with CD40L can present whole proteins representing T-cell neoantigens. This result extends those of other investigators who have shown that peptide-pulsed31,57 or RNA-transfected24 CD40L-activated human B cells can induce primary CD8+ T-cell responses. Notably, the studies presented herein suggest a practical means for recruiting the critical CD4+ T-cell component for cancer immunotherapy strategies that employ MHC class I-restricted tumour-associated peptides. Currently, most studies rely on pan MHC-binding peptides such as PADRE58,59 or hepatitis B core protein60 to generate CD4+ T-cell help that is antigenically unrelated to the specificity of the CD8+ T cells.
Finally, the combination of CD40L activation, which enables relatively long-term growth of antigen-specific B cells and which augments intracellular protein processing, and antigen uptake via the BCR allows for efficient uptake, processing and presentation of whole protein antigens; this is comparable to that observed with mature DC and considerably superior to that seen with non-specific CD40L-activated B cells. The demonstration that CD40L-activated B cells efficiently home to secondary lymphoid organs suggests that these cells express characteristics required for in vivo immunization. These homing studies are consistent with assays performed on human CD40L-activated B cells demonstrating up-regulation of surface proteins important for B-cell homing to lymph nodes.44 These activated human B cells also were capable of stimulating T-cell migration towards the activated APC.44 Experiments to evaluate murine CD40L-activated B cells in vivo are underway.
In summary, the system presented herein not only describes a unique tool for studying growth and function of primary antigen-specific B cells but also supports the hypothesis that the outgrowth of large numbers of highly activated B cells represents a viable and practical complement to DC for cell-based immunotherapy.
Acknowledgments
This work was supported by RO1-ES06086 and PO1-HL68705
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 3.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–8. [PubMed] [Google Scholar]
- 4.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–4. doi: 10.1016/s0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–8. [PubMed] [Google Scholar]
- 6.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–8. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 7.Jefford M, Maraskovsky E, Cebon J, Davis ID. The use of dendritic cells in cancer therapy. Lancet Oncol. 2001;2:343–53. doi: 10.1016/s1470-2045(00)00389-2. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–73. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 9.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21:873–86. doi: 10.1081/cnv-120025091. [DOI] [PubMed] [Google Scholar]
- 10.Svane IM, Pedersen AE, Johansen JS, et al. Vaccination with p53 peptide-pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL-40 and IL-6 as response biomarkers. Cancer Immunol Immunother. 2007;53:1485–99. doi: 10.1007/s00262-007-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol. 2006;25:377–413. doi: 10.1080/08830180600992456. [DOI] [PubMed] [Google Scholar]
- 12.Kyte JA, Gaudernack G. Immuno-gene therapy of cancer with tumour-mRNA transfected dendritic cells. Cancer Immunol Immunother. 2006;55:1432–42. doi: 10.1007/s00262-006-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belz GT, Behrens GM, Smith CM, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belz GT, Heath WR, Carbone FR. The role of dendritic cell subsets in selection between tolerance and immunity. Immunol Cell Biol. 2002;80:463–8. doi: 10.1046/j.1440-1711.2002.01116.x. [DOI] [PubMed] [Google Scholar]
- 15.Nouri-Shirazi M, Thomson AW. Dendritic cells as promoters of transplant tolerance. Expert Opin Biol Ther. 2006;6:325–39. doi: 10.1517/14712598.6.4.325. [DOI] [PubMed] [Google Scholar]
- 16.Tarbell KV, Petit L, Zuo X, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–61. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–40. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 19.Schnurr M, Chen Q, Shin A, et al. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105:2465–72. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- 20.Schultze JL, Grabbe S, von Bergwelt-Baildon MS. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004;25:659–64. doi: 10.1016/j.it.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Coughlin CM, Vonderheide RH. Targeting adult and pediatric cancers via cell-based vaccines and the prospect of activated B lymphocytes as a novel modality. Cancer Biol Ther. 2003;2:466–70. doi: 10.4161/cbt.2.5.445. [DOI] [PubMed] [Google Scholar]
- 22.Trojan A, Schultze JL, Witzens M, et al. Immunoglobulin framework-derived peptides function as cytotoxic T-cell epitopes commonly expressed in B-cell malignancies. Nat Med. 2000;6:667–72. doi: 10.1038/76243. [DOI] [PubMed] [Google Scholar]
- 23.Kondo E, Topp MS, Kiem HP, et al. Efficient generation of antigen-specific cytotoxic T cells using retrovirally transduced CD40-activated B cells. J Immunol. 2002;169:2164–71. doi: 10.4049/jimmunol.169.4.2164. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 25.Maecker B, von Bergwelt-Baildon MS, Sherr DH, Nadler LM, Schultze JL. Identification of a new HLA-A*0201-restricted cryptic epitope from CYP1B1. Int J Cancer. 2005;115:333–6. doi: 10.1002/ijc.20906. [DOI] [PubMed] [Google Scholar]
- 26.Maecker B, Sherr DH, Vonderheide RH, et al. The shared tumor-associated antigen cytochrome P450 1B1 is recognized by specific cytotoxic T cells. Blood. 2003;102:3287–94. doi: 10.1182/blood-2003-05-1374. [DOI] [PubMed] [Google Scholar]
- 27.Van Voorhis WC, Hair LS, Steinman RM, Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982;155:1172–87. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez JA, Bioley G, Turtle CJ, et al. Single step enrichment of blood dendritic cells by positive immunoselection. J Immunol Methods. 2003;274:47–61. doi: 10.1016/s0022-1759(02)00429-5. [DOI] [PubMed] [Google Scholar]
- 29.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonderheide RH, Anderson KS, Hahn WC, Butler MO, Schultze JL, Nadler LM. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin Cancer Res. 2001;7:3343–8. [PubMed] [Google Scholar]
- 31.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–25. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 32.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 33.Abbas AK, Haber S, Rock KL. Antigen presentation by hapten-specific B lymphocytes. II. Specificity and properties of antigen-presenting B lymphocytes, and function of immunoglobulin receptors. J Immunol. 1985;135:1661–7. [PubMed] [Google Scholar]
- 34.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–93. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 35.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–60. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 36.Faassen AE, Dalke DP, Berton MT, Warren WD, Pierce SK. CD40-CD40 ligand interactions stimulate B cell antigen processing. Eur J Immunol. 1995;25:3249–55. doi: 10.1002/eji.1830251208. [DOI] [PubMed] [Google Scholar]
- 37.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 38.Banchereau J, de Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70–2. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 39.Foote LC, Marshak-Rothstein A, Rothstein TL. Tolerant B lymphocytes acquire resistance to Fas-mediated apoptosis after treatment with interleukin 4 but not after treatment with specific antigen unless a surface immunoglobulin threshold is exceeded. J Exp Med. 1998;187:847–53. doi: 10.1084/jem.187.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–9. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 41.Croft M, Dubey C. Accessory molecule and costimulation requirements for CD4 T cell response. Crit Rev Immunol. 1997;17:89–118. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- 42.Schuurhuis DH, Fu N, Ossendorp F, Melief CJ. Ins and outs of dendritic cells. Int Arch Allergy Immunol. 2006;140:53–72. doi: 10.1159/000092002. [DOI] [PubMed] [Google Scholar]
- 43.McHeyzer-Williams LJ, Cool M, McHeyzer-Williams MG. Antigen-specific B cell memory: expression and replenishment of a novel b220(-) memory b cell compartment. J Exp Med. 2000;191:1149–66. doi: 10.1084/jem.191.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Bergwelt-Baildon M, Shimabukuro-Vornhagen A, Popov A, et al. CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood. 2006;107:2786–9. doi: 10.1182/blood-2004-01-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–6. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 46.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–33. [PubMed] [Google Scholar]
- 47.Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–17. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heath WR, Belz GT, Behrens GM, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 49.MartIn-Fontecha A, Sebastiani S, Hopken UE, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–21. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thurner B, Roder C, Dieckmann D, et al. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 51.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984;160:1102–13. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macaulay AE, DeKruyff RH, Goodnow CC, Umetsu DT. Antigen-specific B cells preferentially induce CD4+ T cells to produce IL-4. J Immunol. 1997;158:4171–9. [PubMed] [Google Scholar]
- 53.Casten LA, Kaumaya P, Pierce SK. Enhanced T cell responses to antigenic peptides targeted to B cell surface Ig, Ia, or class I molecules. J Exp Med. 1988;168:171–80. doi: 10.1084/jem.168.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Ham M, van Lith M, Lillemeier B, et al. Modulation of the major histocompatibility complex class II-associated peptide repertoire by human histocompatibility leukocyte antigen (HLA)-DO. J Exp Med. 2000;191:1127–36. doi: 10.1084/jem.191.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seldin MF, Rich RR, Dupont B. Human immune responses to hapten-conjugated cells. II. The roles of autologous and allogeneic histocompatibility determinants in proliferative responses in vitro. J Immunol. 1979;122:1828–33. [PubMed] [Google Scholar]
- 56.Krasteva M, Peguet-Navarro J, Moulon C, Courtellemont P, Redziniak G, Schmitt D. In vitro primary sensitization of hapten-specific T cells by cultured human epidermal Langerhans cells – a screening predictive assay for contact sensitizers. Clin Exp Allergy. 1996;26:563–70. [PubMed] [Google Scholar]
- 57.Jaiswal AI, Croft M. CD40 ligand induction on T cell subsets by peptide-presenting B cells: implications for development of the primary T and B cell response. J Immunol. 1997;159:2282–91. [PubMed] [Google Scholar]
- 58.Hung CF, Tsai YC, He L, Wu TC. DNA Vaccines Encoding Ii-PADRE Generates Potent PADRE-specific CD4(+) T-Cell Immune Responses and Enhances Vaccine Potency. Mol Ther. 2007;15:1211–9. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wierecky J, Muller MR, Wirths S, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910–8. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]
- 60.Graff-Dubois S, Faure O, Gross DA, et al. Generation of CTL recognizing an HLA-A*0201-restricted epitope shared by MAGE-A1, -A2, -A3, -A4, -A6, -A10, and -A12 tumor antigens: implication in a broad-spectrum tumor immunotherapy. J Immunol. 2002;169:575–80. doi: 10.4049/jimmunol.169.1.575. [DOI] [PubMed] [Google Scholar]