Abstract
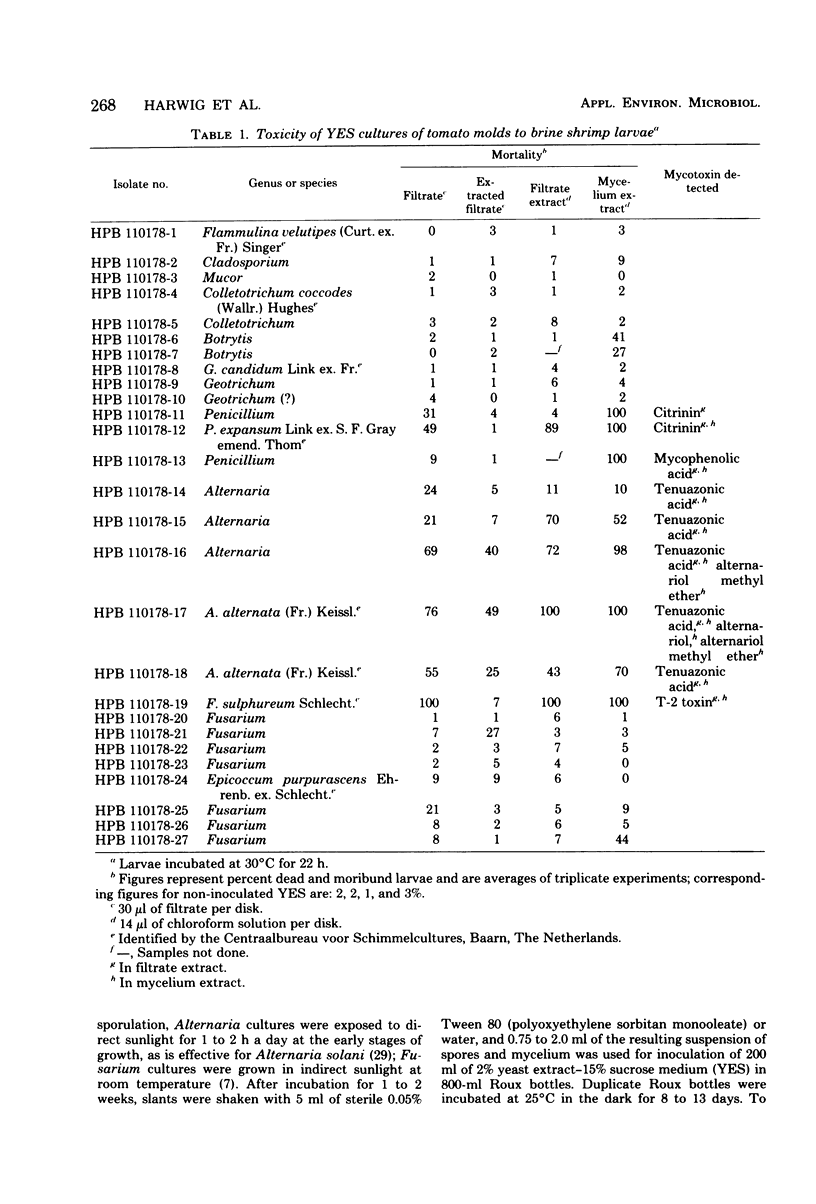
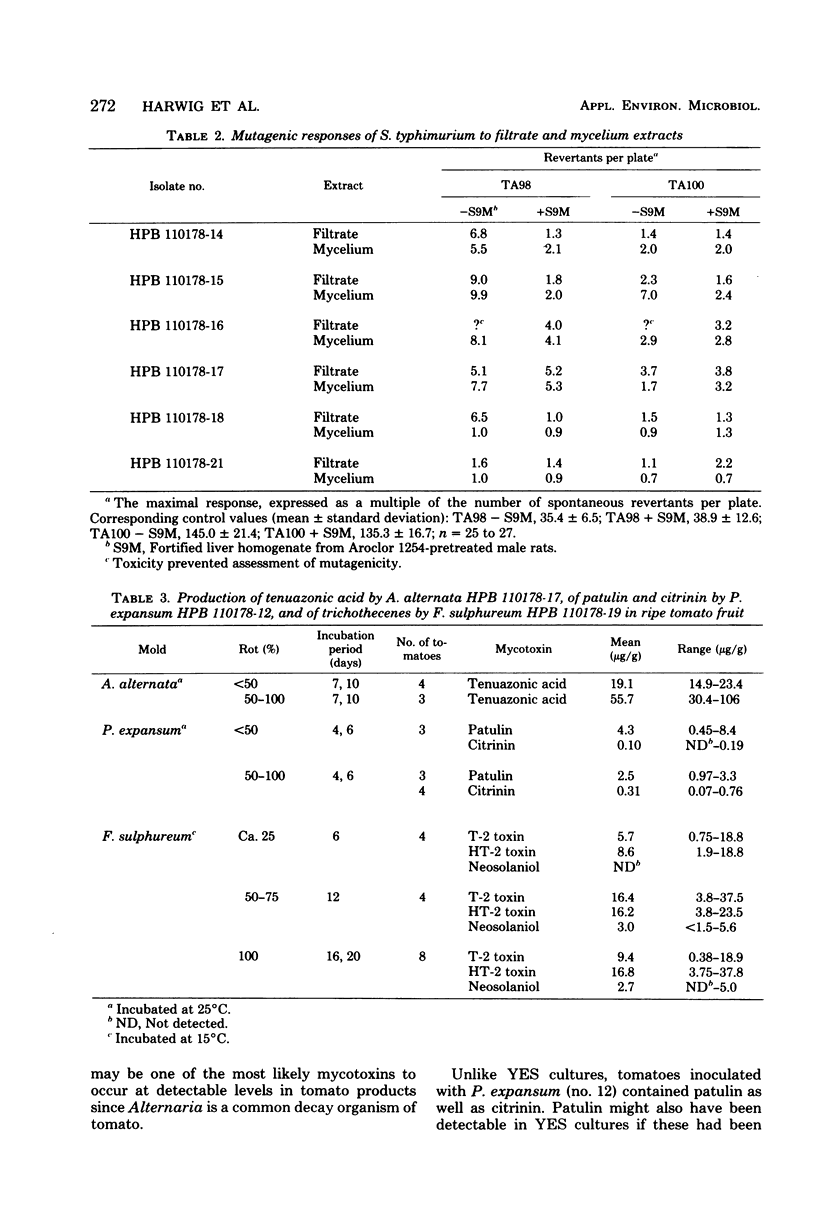
Among 27 mold isolates from decaying tomatoes, culture filtrates or ethyl acetate extracts of 8 isolates grown in yeast extract-sucrose medium were markedly toxic (mortality, greater than 50%) to brine shrimp larvae. The toxicity of six of these isolates could be attributed to the presence of citrinin, tenuazonic acid, or T-2 toxin. Ethyl acetate extracts of five Alternaria isolates and one Fusarium isolate were mutagenic for Salmonella typhimurium strains. In ripe tomatoes inoculated with toxin-producing isolates and incubated at 25 degrees C, one Alternaria alternata isolate produced tenuazonic acid in seven of seven tomatoes at levels of up to 106 micrograms/g and alternariol methyl ether in one of the seven tomatoes at 0.8 microgram/g. Another A. alternata isolate produced tenuazonic acid or alternariol methyl ether at much lower levels in only three of seven tomatoes. Patulin and citrinin were produced by a Penicillium expansum isolate at levels of up to 8.4 and 0.76 microgram/g, respectively. In tomatoes incubated at 15 degrees C, a Fusarium sulphureum isolate produced T-2 toxin, HT-2 toxin, and neosolaniol at levels of up to 37.5, 37.8 and 5.6 micrograms/g, respectively. If these mycotoxins are thermostable, they may occur at detectable levels in tomato products whenever partially moldy tomatoes are used as raw material.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Barkai-Golan R. Species of Penicillium causing decay of stored fruits and vegetables in Israel. Mycopathol Mycol Appl. 1974 Oct 15;54(1):141–145. doi: 10.1007/BF02055983. [DOI] [PubMed] [Google Scholar]
- Bjeldanes L. F., Chang G. W., Thomson S. V. Detection of mutagens produced by fungi with the Salmonella typhimurium assay. Appl Environ Microbiol. 1978 Jun;35(6):1150–1154. doi: 10.1128/aem.35.6.1150-1154.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R. F., Coxon D. T., Levett G. Toxicity of fatty acids in assays for mycotoxins using the brine shrimp (Artemia salina). Food Cosmet Toxicol. 1974 Apr;12(2):233–235. doi: 10.1016/0015-6264(74)90369-1. [DOI] [PubMed] [Google Scholar]
- Davis N. D., Diener U. L., Morgan-Jones G. Tenuazonic acid production by Alternaria alternata and Alternaria tenuissima isolated from cotton. Appl Environ Microbiol. 1977 Aug;34(2):155–157. doi: 10.1128/aem.34.2.155-157.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank H. K., Orth R., Figge A. Patulin in Lebensmitteln pflanzlicher Herkunft. 2. Verschiedene Obstarten, Gemüse und daraus hergestellte Produkte. Z Lebensm Unters Forsch. 1977 Feb 25;163(2):111–114. doi: 10.1007/BF01126030. [DOI] [PubMed] [Google Scholar]
- Harvan D. J., Pero R. W. Gas chromatographic analysis of the Alternaria metabolite, tenuazonic acid. J Chromatogr. 1974 Dec 4;101(1):222–224. doi: 10.1016/s0021-9673(01)94755-7. [DOI] [PubMed] [Google Scholar]
- Harwig J., Scott P. M. Brine shrimp (Artemia salina L.) larvae as a screening system for fungal toxins. Appl Microbiol. 1971 Jun;21(6):1011–1016. doi: 10.1128/am.21.6.1011-1016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczka E. A., Gitterman C. O., Dulaney E. L., Smith M. C., Hendlin D., Woodruff H. B., Folkers K. Discovery of inhibitory activity of tenuazonic acid for growth of human adenocarcinoma-1. Biochem Biophys Res Commun. 1964;14:54–57. doi: 10.1016/0006-291x(63)90210-9. [DOI] [PubMed] [Google Scholar]
- Meronuck R. A., Steele J. A., Mirocha C. J., Christensen C. M. Tenuazonic acid, a toxic produced by Alternaria alternata. Appl Microbiol. 1972 Mar;23(3):613–617. doi: 10.1128/am.23.3.613-617.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero R. W., Posner H., Blois M., Harvan D., Spalding J. W. Toxicity of metabolites produced by the "Alternaria". Environ Health Perspect. 1973 Jun;4:87–94. doi: 10.1289/ehp.730487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B., Dutt B. L. Inducing sporulation in Alternaria solani. II. Effect of light. Mycopathol Mycol Appl. 1974 Oct 15;54(1):47–54. doi: 10.1007/BF02055972. [DOI] [PubMed] [Google Scholar]
- ROSETT T., SANKHALA R. H., STICKINGS C. E., TAYLOR M. E., THOMAS R. Studies in the biochemistry of micro-organisms. 103. Metabolites of Alternaria tenuis auct; culture filtrate products. Biochem J. 1957 Nov;67(3):390–400. doi: 10.1042/bj0670390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie C. J., Van Rensburg S. J., Van Der Watt J. J., Lübben A. Onyalai--the possible involvement of a mycotoxin produced by Phoma sorghina in the aetiology. S Afr Med J. 1975 Sep 20;49(40):1647–1650. [PubMed] [Google Scholar]
- Scott P. M., Kennedy B., Van Walbeek W. Simplified procedure for the purification of ochratoxin A from extracts of Penicillium viridicatum. J Assoc Off Anal Chem. 1971 Nov;54(6):1445–1447. [PubMed] [Google Scholar]
- Scott P. M., Lawrence J. W., van Walbeek W. Detection of mycotoxins by thin-layer chromatography: application to screening of fungal extracts. Appl Microbiol. 1970 Nov;20(5):839–842. doi: 10.1128/am.20.5.839-842.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. M., Panalaks T., Kanhere S., Miles W. F. Determination of zearalenone in cornflakes and other corn-based foods by thin layer chromatography, high pressure liquid chromatography, and gas-liquid chromatography/high resolution mass spectrometry. J Assoc Off Anal Chem. 1978 May;61(3):593–600. [PubMed] [Google Scholar]
- Smith E. R., Fredrickson T. N., Hadidian Z. Toxic effects of the sodium and the N,N'-dibenzylethylenediamine salts of tenuazonic acid (NSC-525816 and NSC-82260). Cancer Chemother Rep. 1968 Sep;52(5):579–585. [PubMed] [Google Scholar]
- Sphon J. A., Dreifuss P. A., Schulten H. R. Field desorption mass spectrometry of mycotoxins and mycotoxin mixtures, and its application as a screening technique for foodstuffs. J Assoc Off Anal Chem. 1977 Jan;60(1):73–82. [PubMed] [Google Scholar]
- Ueno Y., Kubota K., Ito T., Nakamura Y. Mutagenicity of carcinogenic mycotoxins in Salmonella typhimurium. Cancer Res. 1978 Mar;38(3):536–542. [PubMed] [Google Scholar]
- Ueno Y., Sato N., Ishii K., Sakai K., Tsunoda H. Biological and chemical detection of trichothecene mycotoxins of Fusarium species. Appl Microbiol. 1973 Apr;25(4):699–704. doi: 10.1128/am.25.4.699-704.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner F. C., Marasas W. F., Thiel P. G. Lack of mutagenicity to Salmonella typhimurium of some Fusarium mycotoxins. Appl Environ Microbiol. 1978 Apr;35(4):659–662. doi: 10.1128/aem.35.4.659-662.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R. D., Still P. E., Smalley E. B., Schnoes H. K., Strong F. M. Isolation and partial characterization of a mycotoxin from Penicillium roqueforti. Appl Microbiol. 1973 Jan;25(1):111–114. doi: 10.1128/am.25.1.111-114.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


