Abstract
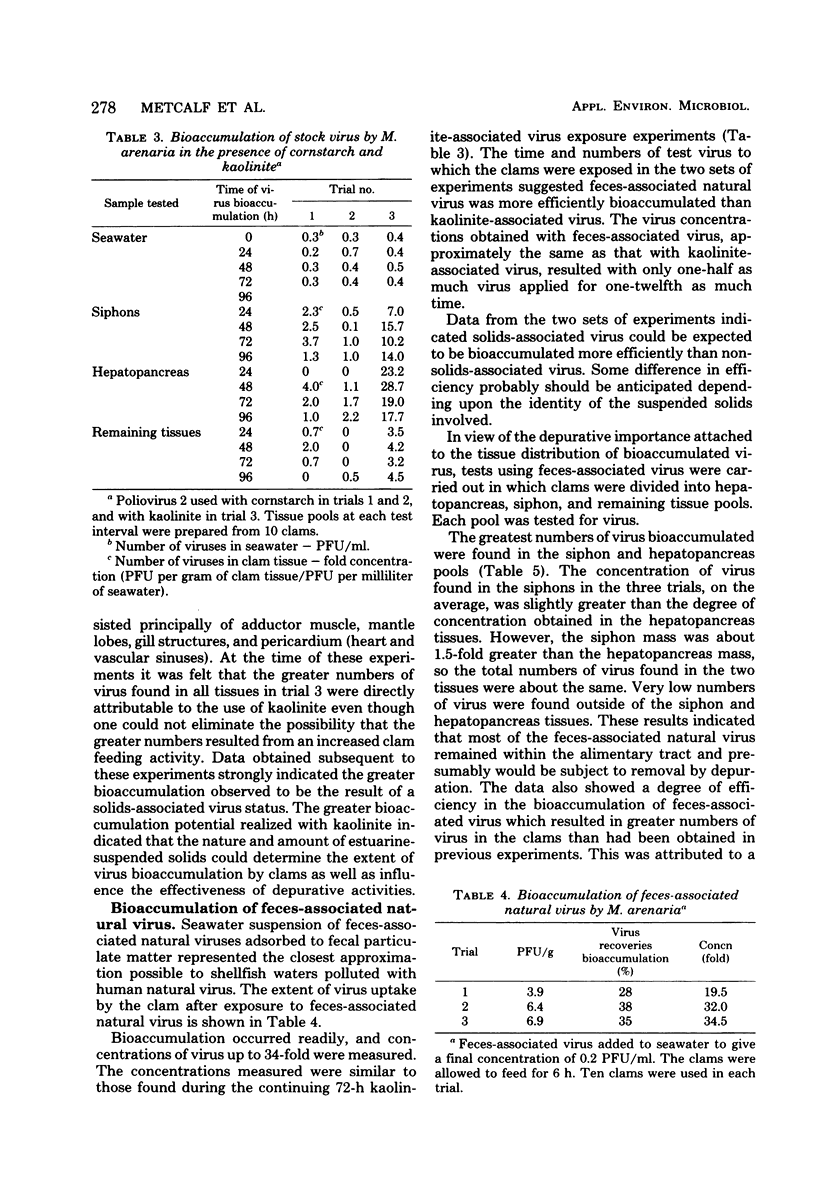
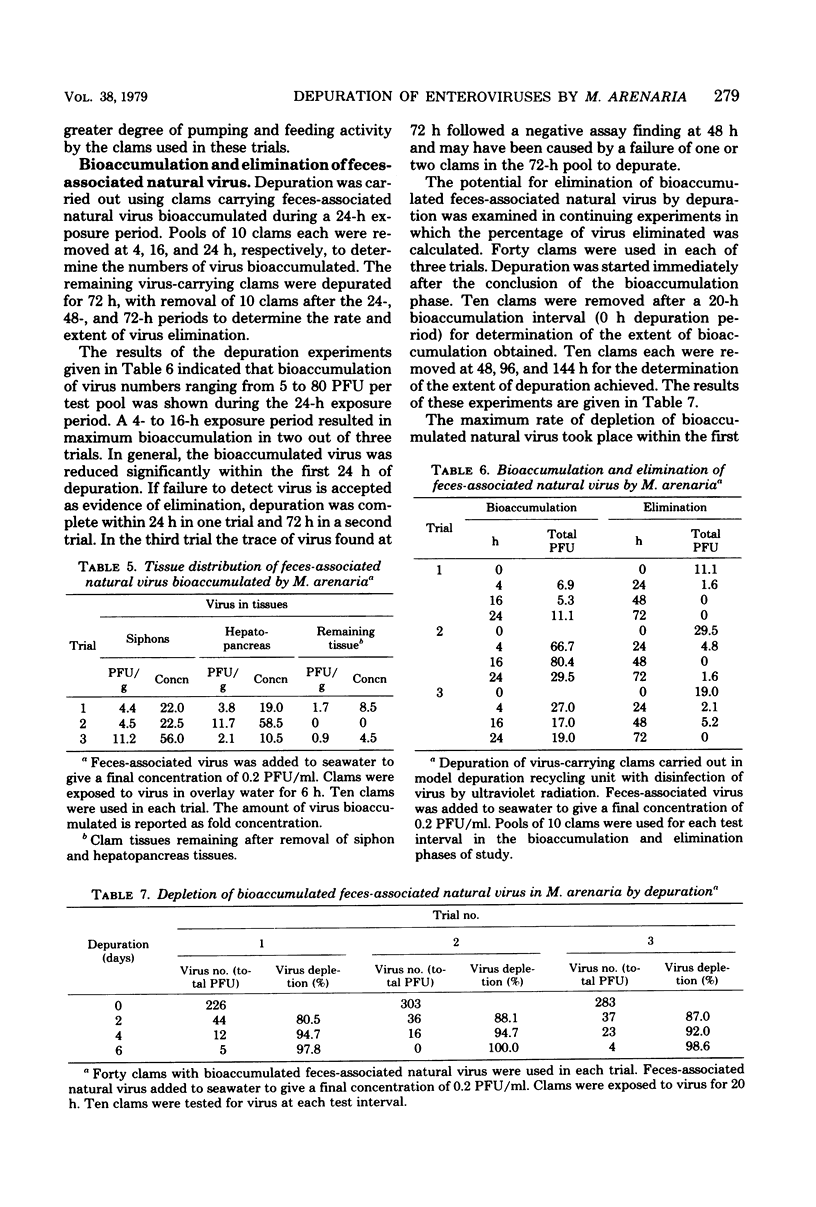
Low levels of feces-associated natural virus, simulating virus numbers estimated to exist in moderately polluted shellfish-growing waters, were used to evaluate the effectiveness of depuration as a virus depletion procedure in soft-shell clams. Depuration effectiveness depended upon the numbers of virus bioaccumulated and whether virus was solids associated. Virus uptake was greatest when viruses were solids associated and pollution levels were equivalent or greater than those likely to be found in grossly polluted growing waters. Virtually all bioaccumulated feces-associated natural virus was deposited within either the hepatopancreas or siphon tissues. Viruses usually were eliminated within a 24- to 48-h depuration period. Dependence upon depuration of clams to elimate health hazards of virus etiology involved a risk factor not measureable in the study. The greatest reduction of health risks would come from the routine depuration of clams harvested from growing waters of good sanitary quality.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCISZ W., KELLY C. B. Self-purification of the soft clam, Mya arenaria. Public Health Rep. 1955 Jun;70(6):605–614. [PMC free article] [PubMed] [Google Scholar]
- Appleton H., Pereira M. S. A possible virus aetiology in outbreaks of food-poisoning from cockles. Lancet. 1977 Apr 9;1(8015):780–781. doi: 10.1016/s0140-6736(77)92960-9. [DOI] [PubMed] [Google Scholar]
- Canzonier W. J. Accumulation and elimination of coliphage S-13 by the hard clam, Mercenaria mercenaria. Appl Microbiol. 1971 Jun;21(6):1024–1031. doi: 10.1128/am.21.6.1024-1031.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- Di Girolamo R., Liston J., Matches J. Ionic bonding, the mechanism of viral uptake by shellfish mucus. Appl Environ Microbiol. 1977 Jan;33(1):19–25. doi: 10.1128/aem.33.1.19-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo R., Liston J., Matches J. Uptake and elimination of poliovirus by West Coast oysters. Appl Microbiol. 1975 Feb;29(2):260–264. doi: 10.1128/am.29.2.260-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDSTROEM C. E., LYCKE E. AN EXPERIMENTAL STUDY ON OYSTERS AS VIRUS CARRIERS. Am J Hyg. 1964 Mar;79:134–142. doi: 10.1093/oxfordjournals.aje.a120369. [DOI] [PubMed] [Google Scholar]
- Hamblet F. E., Hill W. F., Jr, Akin E. W., Benton W. H. Oysters and human viruses: effect of seawater turbidity on poliovirus uptake and elimination. Am J Epidemiol. 1969 May;89(5):562–571. doi: 10.1093/oxfordjournals.aje.a120969. [DOI] [PubMed] [Google Scholar]
- Hoff J. C., Becker R. C. The accumulation and elimination of crude and clarified poliovirus suxpensions by shellfish. Am J Epidemiol. 1969 Jul;90(1):53–61. doi: 10.1093/oxfordjournals.aje.a121049. [DOI] [PubMed] [Google Scholar]
- Koff R. S., Grady G. F., Chalmers T. C., Mosley J. W., Swartz B. L. Viral hepatitis in a group of Boston hospitals. 3. Importance of exposure to shellfish in a nonepidemic period. N Engl J Med. 1967 Mar 30;276(13):703–710. doi: 10.1056/NEJM196703302761301. [DOI] [PubMed] [Google Scholar]
- Liu O. C., Seraichekas H. R., Murphy B. L. Viral depuration of the Northern quahaug. Appl Microbiol. 1967 Mar;15(2):307–315. doi: 10.1128/am.15.2.307-315.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. R., Presnell M. W., Akin E. W., Cummins J. M., Liu O. C. Accumulation and elimination of poliovirus by the eastern oyster. Am J Epidemiol. 1966 Jul;84(1):40–50. doi: 10.1093/oxfordjournals.aje.a120626. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Ho H. H., Riggs J. L., Lennette E. H. Comparative sensitivity of various cell culture systems for isolation of viruses from wastewater and fecal samples. Appl Environ Microbiol. 1978 Sep;36(3):480–486. doi: 10.1128/aem.36.3.480-486.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraichekas H. R., Brashear D. A., Barnick J. A., Carey P. F., Liu O. C. Viral depuration by assaying individual shellfish. Appl Microbiol. 1968 Dec;16(12):1865–1871. doi: 10.1128/am.16.12.1865-1871.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. H., Wallis C., Ward C. H. Inactivation of clay-associated bacteriophage MS-2 by chlorine. Appl Environ Microbiol. 1977 Feb;33(2):385–391. doi: 10.1128/aem.33.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


