Abstract
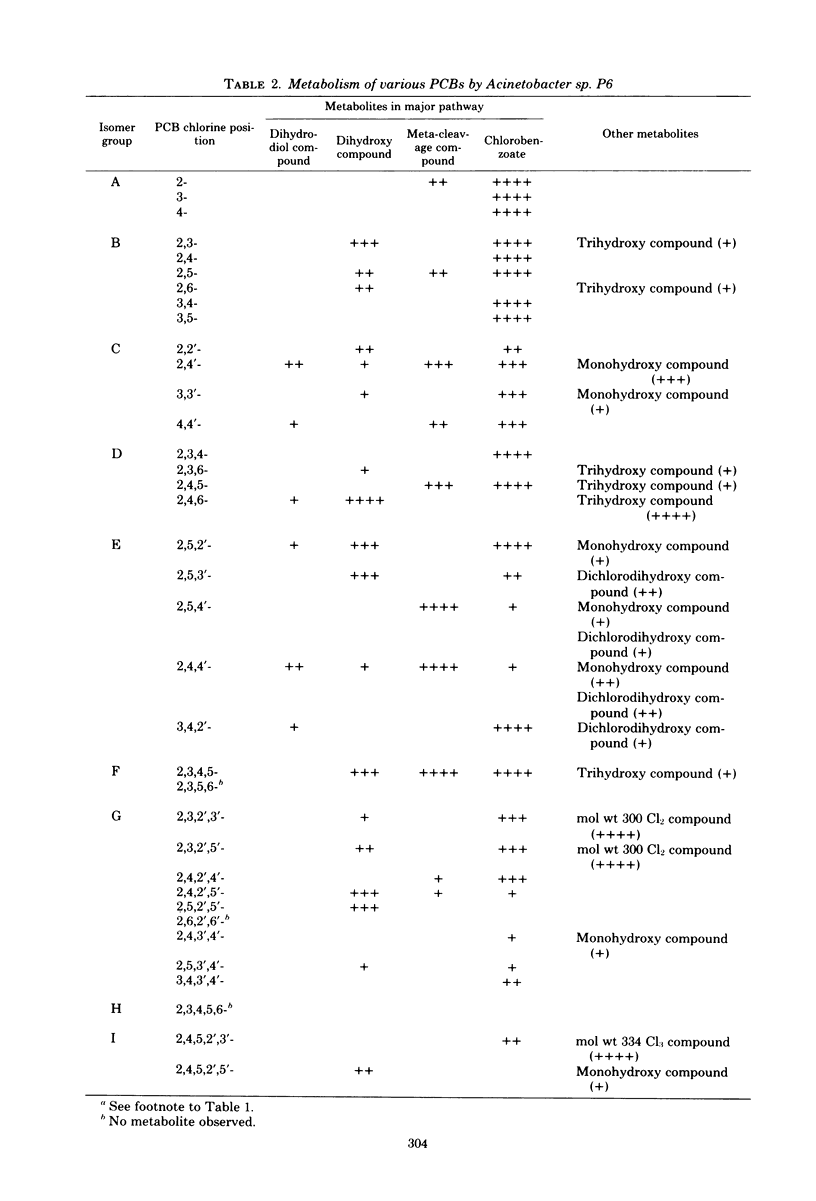
Of 36 pure isomers (chlorine numbers 1 to 5) of polychlorinated biphenyls examined, 23 compounds were metabolized by Alcaligenes sp. strain Y42, and 33 compounds were metabolized by Acinetobacter sp. strain P6. The major pathway of many polychlorinated biphenyl isomers examined was considered to proceed through 2',3'-dihydro-2',3'-diol compounds, concomitant dehydrogenated 2',3'-dihydroxy compounds, subsequently the 1',2'-meta-cleavage compounds (chlorinated derivatives of 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acids), and then chlorobenzoic acids. The meta-cleavage products were usually converted to chlorobenzoic acids upon further incubation in many polychlorinated biphenyls, but they accumulated specifically in the metabolism of 2,4'-, 2,4,4'-, and 2,5,4'-chlorobiphenyls, which are all chlorinated at the 2,4'-position in the molecules in common. Dihydroxy compounds accumulated mainly in the metabolism of 2,6-, 2,3,6-, 2,4,2',5'-, 2,5,2',5'-, and 2,4,5,2',5'-chlorobiphenyls by Acinetobacter sp. P6. The 2,3,2',3'-, 2,3,2',5'-, and 2,4,5,2',3'-chlorobiphenyls, which are chlorinated at the 2,3-position of one of the rings, were metabolized in a different fashion. Two major metabolites of a chlorobenzoic acid and an unknown compound accumulated always in the metabolism of this group of polychlorinated biphenyls. 2,4,6-Trichlorobiphenyl was metabolized quite differently between the two organisms. Alcaligenes sp. Y42 metabolized this compound very slowly to trichlorobenzoic acid by the major oxidative route. In contrast, Acinetobacter sp. P6 metabolized it to a trihydroxy compound via a dihydroxy compound.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Bollag J. M. Microbial transformation of pesticides. Adv Appl Microbiol. 1974;18(0):75–130. doi: 10.1016/s0065-2164(08)70570-7. [DOI] [PubMed] [Google Scholar]
- Crawford R. L., McCoy E., Harkin J. M., Kirk T. K., Obst J. R. Degradation of methoxylated benzoic acids by a Nocardia from a lignin-rich environment: significance to lignin degradation and effect of chloro substituents. Appl Microbiol. 1973 Aug;26(2):176–184. doi: 10.1128/am.26.2.176-184.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. DDT metabolites and analogs: ring fission by Hydrogenomonas. Science. 1970 Oct 2;170(3953):91–92. doi: 10.1126/science.170.3953.91. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Matsumura F. Microbial metabolism of polychlorinated biphenyls. Studies on the relative degradability of polychlorinated biphenyl components by Alkaligenes sp. J Agric Food Chem. 1976 Mar-Apr;24(2):251–256. doi: 10.1021/jf60204a002. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Tonomura K., Kamibayashi A. Effect of chlorine substitution on the biodegradability of polychlorinated biphenyls. Appl Environ Microbiol. 1978 Feb;35(2):223–227. doi: 10.1128/aem.35.2.223-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Cardini G. E., Maseles F. C., Kallio R. E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Hass J. R., Jao L. T., Wilson N. K., Matthews H. B. Metabolism of 4-chlorobiphenyl and 4,4'-dichlorobiphenyl in the rat: qualitative and quantitative aspects. J Agric Food Chem. 1977 Nov-Dec;25(6):1330–1333. doi: 10.1021/jf60214a005. [DOI] [PubMed] [Google Scholar]
- Hutzinger O., Nash D. M., Safe S., DeFreitas A. S., Norstrom R. J., Wildish D. J., Zitko V. Polychlorinated biphenyls: metabolic behavior of pure isomers in pigeons, rats, and brook trout. Science. 1972 Oct 20;178(4058):312–314. doi: 10.1126/science.178.4058.312. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Kutsuna M., Someda K., Morita K., Yamanouchi Y., Kurimoto T., Kawamura Y., Matsumura H. [Ischemic cerebral symptoms after subarachnoid hemorrhage due to aneurysmal rupture (author's transl)]. No Shinkei Geka. 1978 Jun;6(6):543–548. [PubMed] [Google Scholar]
- Reiner A. M., Hegeman G. D. Metabolism of benzoic acid by bacteria. Accumulation of (-)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by mutant strain of Alcaligenes eutrophus. Biochemistry. 1971 Jun 22;10(13):2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- Safe S., Hutzinger O., Jones D. The mechanism of chlorobiphenyl metabolism. J Agric Food Chem. 1975 Sep-Oct;23(5):851–853. doi: 10.1021/jf60201a039. [DOI] [PubMed] [Google Scholar]
- Safe S., Ruzo L. O. The metabolism of 4-chlorobiphenyl in the pig. Can J Physiol Pharmacol. 1975 Jun;53(3):392–396. doi: 10.1139/y75-056. [DOI] [PubMed] [Google Scholar]
- Tucker E. S., Saeger V. W., Hicks O. Activated sludge primary biodegradation of polychlorinated biphenyls. Bull Environ Contam Toxicol. 1975 Dec;14(6):705–713. doi: 10.1007/BF01685246. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Yoshimura H. Metabolic studies on polychlorinated biphenyls. 3. Complete structure and acute toxicity of the metabolites of 2,4,3',4'-tetrachlorobiphenyl. Chem Pharm Bull (Tokyo) 1973 Oct;21(10):2237–2242. doi: 10.1248/cpb.21.2237. [DOI] [PubMed] [Google Scholar]


