Abstract
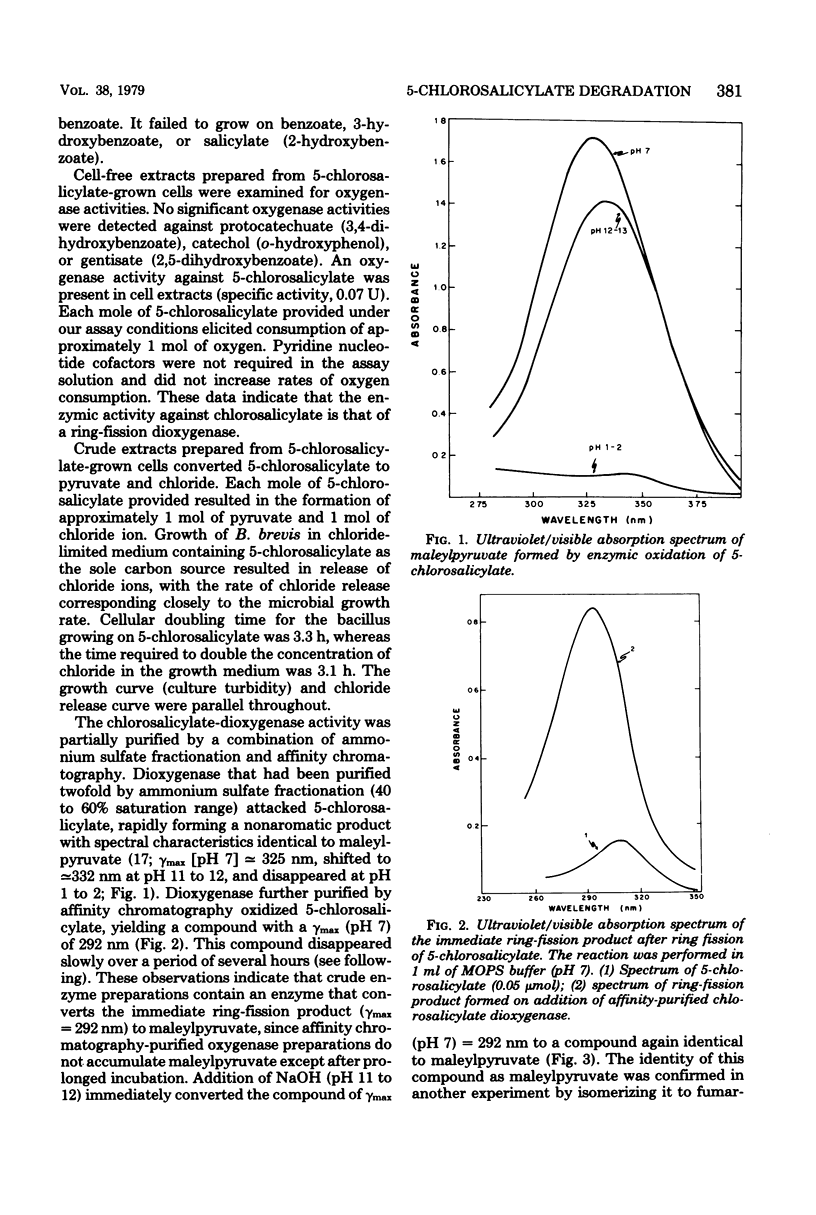
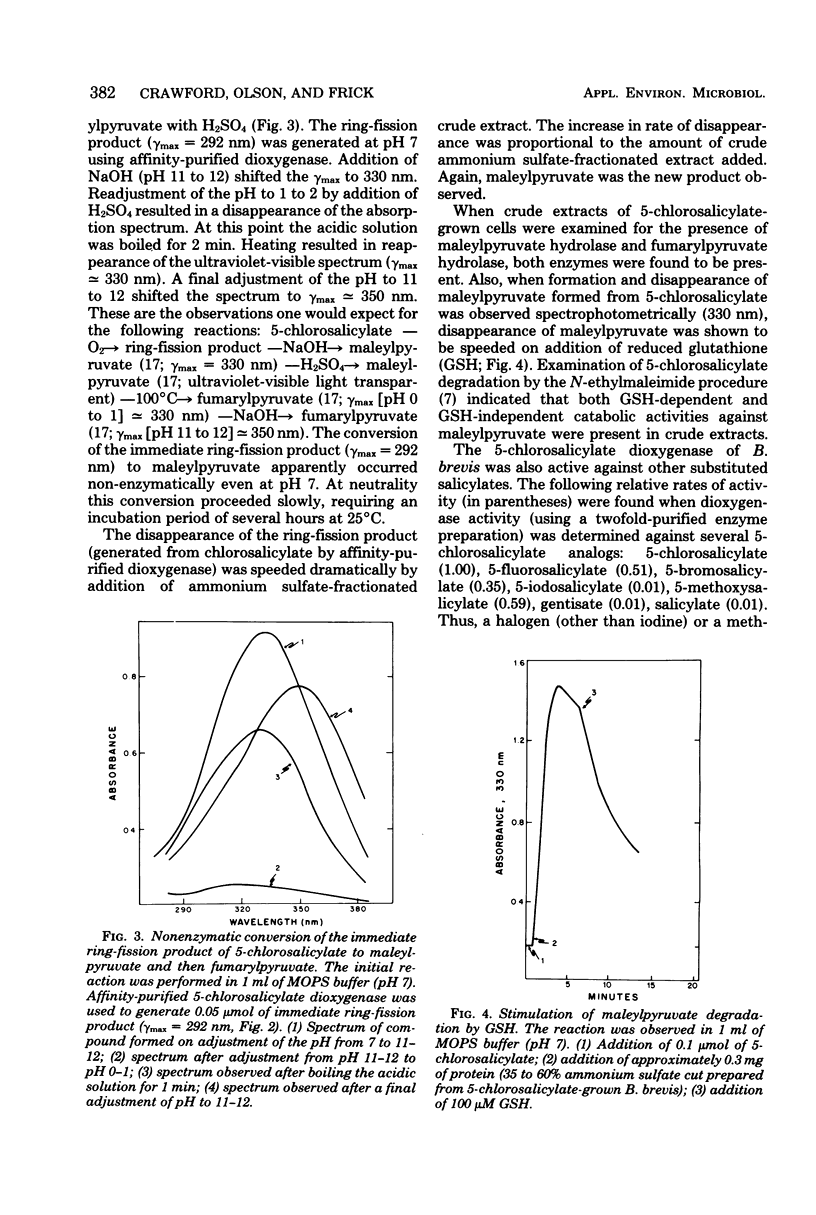
A strain of Bacillus brevis isolated from a polluted section of the Mississippi River was shown to utilize 5-chloro-2-hydroxybenzoate (5-chlorosalicylate) as a sale source of carbon and energy. Enzymic analyses of cell-free extracts prepared from 5-chlorosalicylate-grown cells demonstrated that the initial step in the pathway involved cleavage of the aromatic ring between C1 and C2 by a specific 5-chlorosalicylate 1,2-dioxygenase. Loss of chloride from the growth substrate occurred after ring fission and was probably enzyme mediated. An intermediate chlorolactone apparently lost chloride by enzymatic hydrolysis with formation of maleylpyruvate. Maleylpyruvate was further degraded by both glutathione-dependent and glutathione-independent mechanisms, with these reactions being identical to the terminal reactions of the gentisate pathway. It was suggested that this novel 5-chlorosalicylate pathway may have evolved by recruitment of enzymes from an ancestral gentisate pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collinsworth W. L., Chapman P. J., Dagley S. Stereospecific enzymes in the degradation of aromatic compounds by pseudomonas putida. J Bacteriol. 1973 Feb;113(2):922–931. doi: 10.1128/jb.113.2.922-931.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L. Degradation of 3-hydroxybenzoate by bacteria of the genus Bacillus. Appl Microbiol. 1975 Sep;30(3):439–444. doi: 10.1128/am.30.3.439-444.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Frick T. D. Rapid spectrophotometric differentiation between glutathione-dependent and glutathione-independent gentisate and homogentisate pathways. Appl Environ Microbiol. 1977 Aug;34(2):170–174. doi: 10.1128/aem.34.2.170-174.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Hutton S. W., Chapman P. J. Purification and properties of gentisate 1,2-dioxygenase from Moraxella osloensis. J Bacteriol. 1975 Mar;121(3):794–799. doi: 10.1128/jb.121.3.794-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L. Novel pathway for degradation of protocatechuic acid in Bacillus species. J Bacteriol. 1975 Feb;121(2):531–536. doi: 10.1128/jb.121.2.531-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., GIBSON D. T. THE BACTERIAL DEGRADATION OF CATECHOL. Biochem J. 1965 May;95:466–474. doi: 10.1042/bj0950466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley S. Catabolism of aromatic compounds by micro-organisms. Adv Microb Physiol. 1971;6(0):1–46. doi: 10.1016/s0065-2911(08)60066-1. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN S. The enzymic conversion of 4-fluorophenylalanine to tyrosine. Biochim Biophys Acta. 1961 Aug 19;51:619–621. doi: 10.1016/0006-3002(61)90632-1. [DOI] [PubMed] [Google Scholar]
- LACK L. The enzymic oxidation of gentisic acid. Biochim Biophys Acta. 1959 Jul;34:117–123. doi: 10.1016/0006-3002(59)90239-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Que L., Jr Extradiol cleavage of o-aminophenol by pyrocatechase. Biochem Biophys Res Commun. 1978 Sep 14;84(1):123–129. doi: 10.1016/0006-291x(78)90272-3. [DOI] [PubMed] [Google Scholar]


