Abstract
The time course of G-protein-coupled responses is largely determined by the kinetics of GTP hydrolysis by the G protein α subunit, which is accelerated by interaction with regulator of G-protein signaling (RGS) proteins. Light responses of ON-bipolar cells of the vertebrate retina require rapid inactivation of the G protein Gαo, which is activated in the dark by metabotropic glutamate receptor, mGluR6, in their dendritic tips. It is not yet known, however, which RGS protein(s) might be responsible for rapid inactivation kinetics. By immunofluorescence and co-immunoprecipitation, we have identified complexes of the Gαo-selective RGS proteins RGS7 and RGS11, with their obligate binding partner, Gβ5, that are localized to the dendritic tips of murine rod and cone ON-bipolar cells, along with mGluR6. Experiments using pre- and post-synaptic markers, and a dissociated bipolar cell preparation, clearly identified the location of these complexes as the ON-bipolar cell dendritic tips and not the adjacent photoreceptor terminals or horizontal cell dendrites. In mice lacking mGluR6, the distribution of RGS11, RGS7 and Gβ5 shifts away from the dendritic tips, implying a functional relationship with mGluR6. The precise co-localization of Gβ5–RGS7 and Gβ5–RGS11 with mGluR6, and the dependence of localization on the presence of mGluR6, suggests that Gβ5–RGS7 and Gβ5–RGS11 function specifically in the mGluR6 signal transduction pathway, where they may stimulate the GTPase activity of Gαo, thus accelerating the ON-bipolar cell light response, in a manner analogous to the acceleration of photoreceptor light responses by the Gβ5–RGS9-1 complex.
Keywords: metabotropic glutamate receptor, mouse, retina, ribbon synapse, synaptic transmission
Introduction
Glutamate release from retinal photoreceptors simultaneously depolarizes OFF-bipolar cells and hyperpolarizes ON-bipolar cells. The inhibitory action of glutamate on ON-bipolar cells is mediated by a unique metabotropic glutamate receptor, mGluR6, located in the ON-bipolar cell dendrites (Nomura et al., 1994). Upon binding glutamate, mGluR6 activates a G-protein pathway that closes a non-selective cation channel, and hyperpolarizes the cell (Schiells & Falk, 1990; Nawy & Jahr, 1991; Yamashita & Wässle, 1991). In response to light, glutamate release by photoreceptors drops, and the mGluR6-coupled Gα subunit, Gαo, is rapidly inactivated, causing the channels to open, thereby depolarizing the ON-bipolar cells (Nawy, 1999; Dhingra et al., 2000; Vardiet al., 2000). In some respects, the mGluR6 transduction pathway is similar to phototransduction, where light activates the Gα subunit of transducin (GαT) leading to the closure of cGMP-gated cation channels and hyperpolarization of the photoreceptor.
The intrinsic rates of GTP hydrolysis by Gαo and GαT are slow, requiring tens of seconds. In outer segments, GαT GTPase activity is accelerated by interaction with the Gβ5L–RGS9 complex. The GTPase accelerating activity of Gβ5L–RGS9 is critical in setting the inactivation kinetics of phototransduction, as mice lacking either RGS9 (Chen et al., 2000) or Gβ5 (Krispel et al., 2003) show normal activation of the outer segment photoresponse, but 10–50-fold slower deactivation than wild-type. It is likely that the rapid response of ON-bipolar cells to light requires a similar GTPase accelerating activity. Like GαT, the rate of GTP hydrolysis by Gαo may be accelerated by interaction with a Gβ5–RGS complex.
Immunoreactivity for Gβ5 has been reported in outer segments and the outer plexiform layer (OPL) of the retina (Zhang et al., 2003). Gβ5 exists as two isoforms, Gβ5S and Gβ5L, that are identical except for 42 amino acids on the amino end of Gβ5L (Watson et al., 1996). The long form (Gβ5L) is retina-specific and found exclusively in outer segments (Watson et al., 1996), whereas the shorter form (Gβ5S), which is present in both retina and brain, is excluded from rod outer segments, but is found in cone outer segments along with Gβ5L (Zhang et al., 2003). Gβ5 is the most divergent in amino acid sequence of the five known mammalian Gβ subunits (von Weizsacker et al., 1992; Watson et al., 1996). Unlike other Gβ subunits, which bind to Gγ subunits, Gβ5 forms complexes instead with the R7 family of RGS (regulator of G protein signaling) proteins that includes RGS6, RGS7, RGS9 and RGS11. The R7-RGS proteins contain a Gγ-like (GGL) domain that mediates binding to Gβ5 (Simonds & Zhang, 2000). They also possess a GTPase-accelerating protein (GAP) domain within a conserved ~120-amino-acid sequence (Popov et al., 1997), through which they promote the intrinsic GTPase activity of Gα subunits.
Here we present evidence that Gβ5–RGS7 and Gβ5–RGS11 are present in ON-bipolar cells where they co-localize with mGluR6 in the dendrites in wild-type animals, but show altered distribution in mice lacking mGluR6, suggesting a similar mechanism of deactivation of both the ON-bipolar cell response and phototransduction.
Methods
Immunohistochemistry
Immunohistochemistry on retina sections and acutely dissociated rod bipolar cells was performed as described previously (Berntson et al., 2003; Morgans et al., 2005). Six adult mice were used and killed by cervical dislocation in accordance with OHSU Institutional Animal Care and Use Committee guidelines. Rabbit polyclonal antibody (R4612) against full-length bovine RGS7 and goat polyclonal antibody against Gβ5 (peptide MATDGLHENETLASLKC) were generated at Bethyl Laboratories (Montgomery, TX, USA). The RGS11 antibody was raised in rabbits against a recombinant polypeptide corresponding to residues 248–471 of mouse RGS11 (Chen et al., 2003). The RGS9 rabbit antiserum was generated against a polypeptide corresponding to amino acids 226–484 of bovine RGS9 (He et al., 1998). Antibodies were used at the following concentrations: Gβ5, 1 : 500–1 : 1000; RGS7, 1 : 500–1 : 1000; RGS9, 1 : 1000–1 : 2000; RGS11, 1 : 1000–1 : 5000; mGluR6 (Morgans et al., 2006), 1 : 100; PKCα (Sigma, St Louis, MO, USA), 1 : 5000; calbindin (Sigma), 1 : 1000; anti-ctbp2/ribeye (BD Biosciences Pharmingen, San Diego, CA, USA), 1 : 5000. Appropriate secondary antibodies were coupled to either CY3 (Jackson Immunochemicals, West Grove, PA, USA) and diluted 1 : 500, or Alexa-488 (Molecular Probes, Eugene, OR, USA) and diluted 1: 2000.
Microscopy
Images of the dissociated bipolar cells were obtained on a Nikon Eclipse E800 fluorescence microscope with a 100×/1.30 oil-immersion objective and Metamorph software. All other images were acquired on a Zeiss LSM 510 confocal microscope with a 63×/1.40 oil-immersion objective. All confocal figures show single optical sections of < 1 μm thickness. For figures, brightness and contrast of images were optimized with Adobe Photoshop 7.0.
Image averaging
The localization of Gβ5 relative to ribeye, calbindin and mGluR6 was determined using ImageJ software (W.S. Rasband, NIH, Bethesda, MD, USA), and a signal averaging method similar to that described by Massey & Mills (1999) and Zhang et al. (2002). Twenty 50× 50-pixel images, centred on Gβ5-immunopositive puncta, were averaged. The average intensity of each label was plotted using the ImageJ RGB_Profiler plugin (Laummonerie and Mutterer, Institut de Biologie Moléculaire des Plantes, Strasbourg, France).
Immunoprecipitation from mouse retina lysate
Affinity-purified anti-Gβ5 or pre-immune IgG (0.6 mg) was coupled to 1 mL AffiGel-15 media (Bio-Rad) in 0.1 M 4-morpholinepropane-sulphonic acid (MOPS), pH 7.5, and used for immunoprecipitations. Freshly dissected mouse retinas were homogenized in homogenization buffer [20 mM HEPES, pH 7.0, 150 mil NaCl, 3 mM MgCl2, 1 mM CaCl2, 1 mM β-ME, 1 mM EDTA, 0.01% NaN3, 0.2% C12E10, protease inhibitors (2 μg/mL aprotinin, 2 μg/mL chymostatin, 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin A, 30 μg/mL trypsin inhibitor, 1.6 mg/mL benzamide, 0.1 μM E64, 167 μM Pefabloc, and phenylmethylsulphonyl fluoride)], sonicated on ice for 60 s and incubated at 4 °C for 1 h with gentle shaking. After centrifugation at 100 000g for 30 min, equal amounts of supernatant were applied to anti-Gβ5 IgG- or pre-immune IgG-coupled columns, washed with homogenization buffer, and immunoprecipitated proteins eluted with CT215 peptide (amino-terminal 16 amino acids of Gβ5) followed by SDS-PAGE and Western blotting.
Western blotting
Retinal extracts were subjected to electrophoresis on precast Novex 4–12% polyacrylmaide gradient gels (Invitrogen, Carlsbad, CA, USA), and then the separated proteins electrophoretically transferred to nitrocellulose membranes, which were probed with different antibodies as previously described (Morgans et al., 2006).
Results
Localization of Gβ5 to ON-bipolar cell dendrites
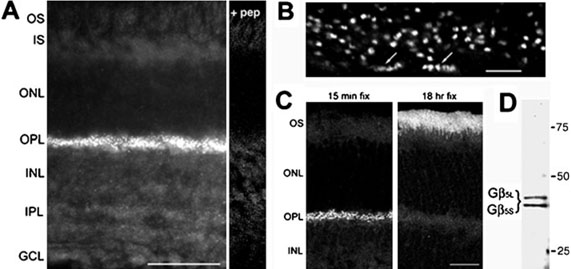
Immunofiuorescent staining of Gβ5 in the mouse retina revealed punctate labeling in the OPL, and faint labeling in both the ON and the OFF strata of the inner plexiform layer (IPL) (Fig. 1A). The immunofiuorescence was blocked by preincubation of the antibody with the peptide immunogen (+ pep, Fig. 1A) demonstrating specificity of the labeling. At high magnification, the OPL labeling could be resolved as pairs of smaller puncta (Fig. 1B) most likely associated with rod photoreceptor terminals. Gβ5 staining associated with cone terminals was also evident (Fig. 1B). Previous work has localized the photoreceptor-specific, long isoform of Gβ5, called Gβ5L, to outer segments (Watson et al., 1996). Despite detecting both Gβ5 and Gβ5L on Western blots of mouse retinal extract (Fig. 1D), we did not observe immunofiuorescent staining of outer segments. The previous study used longer fixation times of 18 h in 4% paraformaldehyde, whereas we fixed for only 15 min. We found that with long fixation times (18 h), staining of outer segments increased dramatically, whereas the OPL staining markedly diminished (Fig. 1C); thus, the two Gβ5 isoforms in the retina, Gβ5L and Gβ5, may be differentially affected by fixation. Light fixation conditions were chosen for analysis of Gβ5 in the OPL.
FIG. 1.
Gβ5 is present in the outer plexiform layer of the retina. (A) Immunofluorescent labeling of a mouse retinal section for Gβ5. All staining is blocked by pre-incubation of the antibody with the peptide immunogen (+ pep). (B) High-power view of Gβ5 labeling in the OPL. Labeling is associated with both rod and cone (arrows) terminals. (C) Fixation dependence of Gβ5 labeling demonstrated with retinas fixed for either 15 min or 18 h in 4% paraformaldehyde. (D) Western blot of mouse retina extract with the Gβ5 antiserum. Scale bars represent 50 μrn in A, 5 μrn in B, and 25 μrn in C. Abbreviations: OS, outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
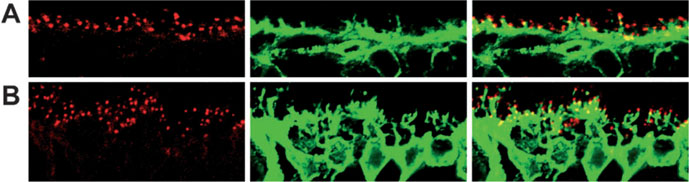
To compare the localization of Gβ5 with ON-bipolar cell dendrites, retina sections were double labeled for Gβ5 and for either Gαo (present in all ON-bipolar cells) or PKCα (a rod bipolar cell marker) (Haverkamp & Wassle, 2000). As observed previously (Dhingra et al., 2000; Haverkamp & Wassle, 2000) both Gαo and PKCα are present both in the dendritic tips and throughout the cell body, whereas Gβ5 staining is strikingly concentrated in the dendritic tips, where its signal coincides with PKCα in rod bipolar cells and with Gαo in both rod and cone ON-bipolar cells (Fig. 2).
FIG. 2.
Gβ5 is located in the tips of ON-bipolar cell dendrites. Mouse retina sections were double labeled for (A) Gβ5 (red) and Gαo (green), and (B) Gβ5 (red) and the rod bipolar cell marker PKCα (green). Scale bar in A represents 10 μm and also applies to B.
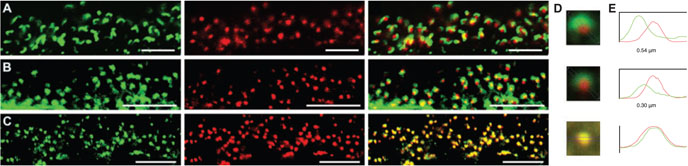
In contrast, Gβ5 staining did not coincide with staining for the presynaptic marker ribeye, a synaptic ribbon protein (Fig. 3A), nor did it coincide with staining for calbindin, a marker for the post-synaptic processes of horizontal cells (Fig. 3B), although it was adjacent to both. Thus, all detectable Gβ5 staining in the OPL seems to be confined to the post-synaptic bipolar cells. This conclusion is further supported by strong co-localization of Gβ5 and mGluR6 (Fig. 3C). To quantify this localization, we averaged twenty 50× 50-pixel images centered on Gβ5 puncta and determined the intensity distribution profile of each marker (Fig. 3D and E). The advantage of this technique is that the centers of intensity distribution profiles can be located with much greater precision than the nominal resolution of the image, given by the width of the Airy disk. We found that the peak Gβ5 signal in immunoreactive puncta was clearly separated from the peak of the ribeye (ribbon) staining by 540 nm, and somewhat closer (340 nm) but still distinct from the peak of calbindin staining (horizontal cell processes). In contrast, the peaks of mGluR6 and Gβ5 staining are within one pixel (125 nm) of one another.
FIG. 3.
Gβ5 is localized post-synaptically in ON-bipolar cell dendrites. (A) Gβ5 (red) and the synaptic ribbon protein ribeye (green), (B) Gβ5 (red) and the horizontal cell marker calbindin (green), or (C) Gβ5 (red) and mGluR6 (green). Areas of co-localization appear yellow. (D) Averaged images centered on the Gβ5 puncta in A–C. (E) Plots of the corresponding channel intensity profiles. The scale bars represent 5 μm in A, and 10 μm in B and C.
RGS7 and RGS11 complexes are present in the OPL
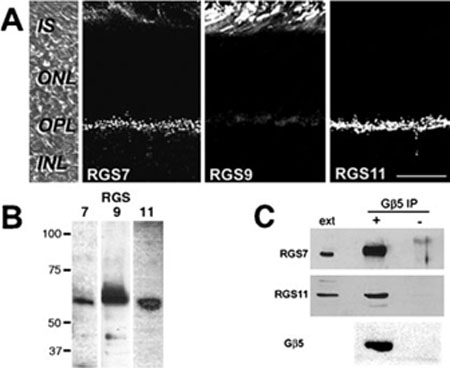
Endogenous Gβ5 typically exists in a tightly bound complex with a GGL domain containing RGS protein; for example, in outer segments Gβ5L is bound to RGS9. The presence of Gβ5 in the OPL suggests that an R7-RGS protein may also be localized in the OPL in a complex with Gβ5. We compared the distribution of RGS7, 9 and 11 in mouse retina by immunofluorescent confocal microscopy (Fig. 4A). RGS6 has been shown previously to be confined to the inner retina (Song et al., 2007). As reported (Zhang et al., 2003), strong RGS9 immunoreactivity was detected in the photoreceptor outer segments, with little staining elsewhere. In contrast, both the RGS7 and the RGS11 antibodies intensely labeled the OPL, with faint RGS7 staining also occurring over the outer segments (Fig. 4A). For both RGS7 and RGS11, bright puncta were labeled throughout the OPL associated with rod and cone terminals, similar to that observed for Gβ5. The specificity of the antisera against RGS7, 9 and 11 was demonstrated with Western blots of mouse retinal extract in which all three antisera labeled bands at the predicted molecular weight (Fig. 4B).
FIG. 4.
RGS7 and RGS11 are localized to the OPL and can be immunopre-cipitated from retinal extracts with Gβ5. (A) Mouse retina sections were labeled by immunofluorescence for RGS7, RGS9 and RGS11. The left panel shows a DIC image of a mouse retina section with the layers indicated. The scale bar represents 20 μm,and applies to all panels. (B) Immunoblots of mouse retinal extract with antisera against RGS7, 9 and 11. (C) Mouse retinal extract was immunoprecipitated with anti-Gβ5 (+) or preimmune serum (−), and then Western blotted for Gβ5, RGS7 and RGS11. Included on the blots were lanes containing retinal extract (ext). Abbreviations: OS, outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer.
Immunoprecipitation experiments (Fig. 4C) confirmed that both RGS7 and RGS11 are tightly associated with Gβ5 in retinal extracts. Mouse retinal extract was passed over columns coupled with either Gβ5 antiserum or preimmune serum. After washing and eluting, the bound proteins were subjected to Western blotting with the Gβ5, RGS7 and RGS11 antisera. Total retinal extract was included on the gel as a positive control. All three antisera detected single bands at the predicted molecular weight in the Gβ5 immunoprecipitation samples, as well as in the total retinal extract, but not in the preimmune samples, indicating a specific association of RGS7 and RGS11 with Gβ5 in the retina.
Co-localization of Gβ5–RGS7 and Gβ5–RGS11 with mGluR6
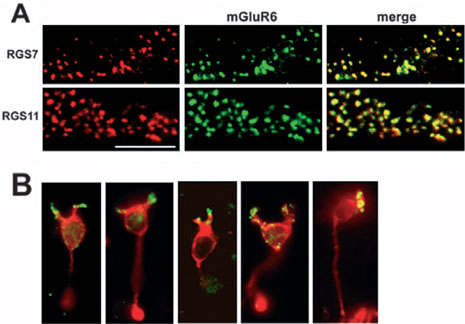
Similar to Gβ5, double labeling for either RGS7 or RGS11 and mGluR6 revealed extensive overlap of the RGS7 and RGS11 puncta with mGluR6, suggestive of localization of RGS7 and RGS11 to ON-bipolar cell dendrites (Fig. 5A).
FIG. 5.
RGS7 and RGS11 co-localize with mGluR6 in the OPL. (A) Mouse retina sections were double labeled for mGluR6 (green) and either RGS7 or RGS11 (red). Areas of co-localization appear yellow. The scale bar represents 10 urn and applies to all panels. (B) Acutely dissociated mouse rod bipolar cells were double labeled for RGS11 (green) and PKCα (red).
To confirm the localization of the complexes to ON-bipolar cell dendrites, RGS11 immunoftuorescence was assessed in acutely dissociated rod bipolar cells (Fig. 5B). Rod bipolar cells, identified by PKCα immunoftuorescence, were found to contain RGS11 immunoftuorescence in their dendrites, confirming the post-synaptic localization of the RSG11 complex to ON-bipolar cells. Similar results were obtained with the Gβ5 antibody (not shown).
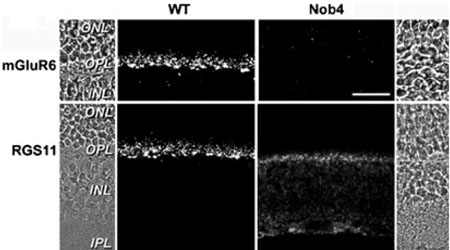
The strict co-localization of Gβ5–RGS7 and Gβ5–RGS11 with mGluR6 in the ON-bipolar cell dendrites suggests that these proteins may be localized by a common mechanism, or may depend on one another for localization. We examined this possibility by comparing the distribution of RGS7, RGS11 and Gβ5 in wild-type and nob4 (mGluR6-deficient) mouse retina sections. The nob4 mouse contains a chemically induced point mutation in the gene encoding mGluR6 (Grm6), resulting, immunohistochemically, in no detectable mGluR6 protein in the retina, and physiologically, in an absence of ganglion cell ON-responses (Pinto et al., 2007). Immunohistochemical comparison of ON-bipolar cell-associated proteins between wild-type and nob4 retinas showed no marked differences in the distributions of PKCα (Pinto et al., 2007), Gαo or nyctalopin (data not shown; Morgans et al., 2006). RGS11 (Fig. 6), on the other hand, showed a striking shift in its staining pattern between the wild-type and the nob4 retinas. A similar alteration in the staining pattern was observed for RGS7 and Gβ5 (data not shown). In the nob4 retina, punctate staining associated with rod terminals was lost from the OPL. Staining associated with cone terminals persisted in the nob4 OPL, but the intensity and punctate appearance of the staining was diminished. In the nob4 retina, all three proteins appeared more diffusely distributed throughout the ON-bipolar cells, as staining was detectable in bipolar cell bodies and in the ON-sublamina of the IPL. These data suggest that mGluR6 is required for restricting the Gβ5–RGS7 and Gβ5–RGS11 complexes to the ON-bipolar cell dendrites.
FIG. 6.
RGS11 is mislocalized in the nob4 retina. Immunofluorescent localization of mGluR6 (top panels) and RGS11 (bottom panels) in wild-type (left) and nob4 (right) retinas. Abbreviations: ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer. The scale bar represents 20 urn and applies to all panels.
Discussion
In ON-bipolar cells, activation of mGluR6 by glutamate leads to the closure of a non-selective cation channel and hyperpolarization of the cell. This response depends on the presence of the heterotrimeric Gprotein, Go, specifically the Gαo1 splice variant of the Gαo subunit (Dhingra et al., 2002), but other components of the pathway have not yet been identified. Data presented here suggest that the Gβ5–RGS7 and Gβ5–RGS11 complexes may also be part of the mGluR6 pathway. RGS7 and RGS11 can be co-immunoprecipitated with Gβ5 from retinal extracts, and Gβ5, RGS7 and RGS11 all co-localize with mGluR6 to the tips of ON-bipolar cell dendrites. In the nob4 mouse, which lacks mGluR6, the Gβ5 complexes are mislocalized, appearing more diffusely distributed through the ON-bipolar cells, suggesting a dependence on mGluR6 for the dendritic localization in the wild-type animal.
Although the downstream targets of Gαo in the retina are not known, its broad distribution throughout ON-bipolar cells suggests that it is likely to act in diverse signaling pathways in these cells, and may interact with distinct modulators and effectors in different subcellular compartments. Gγ13, first identified as the γ subunit of gustducin, a transducin-like G protein expressed by taste receptor cells (Huang et al., 1999), has been proposed to interact with Gαo in ON-bipolar cells. Gγl3 antibodies selectively label ON-bipolar cells in the retina, and similar to Gαo, the staining fills the entire cell from the dendrites to the synaptic terminals (Huang et al., 2003). Likewise, the Gα guanine nucleotide dissociation inhibitor, L7 (pcp-2), fills the entirety of retinal rod bipolar cells (Berrebi et al., 1991). Another potential Gαo modulator is the GAP RET–RGS1 (an isoform of RGS20; Faurobert & Hurley, 1997), which is widely distributed throughout the retina including in photoreceptors, bipolar cells and ganglion cells (Dhingra et al., 2004). It can be co-immunoprecipitated from retinal extracts with Gαo (Dhingra et al., 2004), suggesting that it may regulate Gαo function in one or more of these locations.
In contrast to the above-mentioned Gαo modulator/ effectors, the Gβ5–RGS7 and Gβ5–RGS11 complexes are the only Gαo-interacting proteins identified to date that are confined to the dendritic tips of the ON-bipolar cells, and not broadly distributed through the cell. This restricted localization appears to depend on mGluR6, as it is lost in the nob4 mouse (Fig. 6). The restricted localization of Gβ5–RGS7 and Gβ5–RGS11 would serve to regulate Gαo activity locally, in the vicinity of mGluR6. The precise co-localization with mGluR6 argues that both RGS7-Gβ5 and Gβ5–RGS11 may function specifically in the mGluR6 signal transduction pathway.
Such a role for Gβ5–RGS7 and/or Gβ5–RGS11 complexes in the mGluR6 signal transduction pathway of ON-bipolar cell dendrites would be reminiscent of the role of the Gβ5L–RGS9 complex of photoreceptor outer segments, where it deactivates the Gα subunit of transducin by stimulating GTP hydrolysis. In outer segments, this GTPase accelerating activity is critical in setting the inactivation kinetics of phototransduction (Krispel et al., 2006). Similar to the action of Gβ5–RGS9-1 on GαT, Gβ5–RGS7 and Gβ5–RGS11 complexes have been shown to accelerate GTP hydrolysis by Gαo in heterologous expression systems (Snow et al., 1999; Lan et al., 2000; Hooks et al., 2003). If Gβ5–RGS7 and Gβ5–RGS11 act as GTPase accelerators in ON-bipolar cells, they may be responsible for the rapid deactivation of the ON-bipolar cell response to glutamate, allowing a prompt light response. Spontaneous GTP hydrolysis by Gαo is too slow to account for the observed kinetics of the ON-bipolar cell light response, which reaches its peak amplitude within 100 ms (Berntson & Taylor, 2000). A working model would be that upon illumination and a concomitant drop in glutamate release from photoreceptors, mGluR6 ceases to activate Gαo, and Gβ5–RGS7 and Gβ5–RGS11 located in the vicinity of mGluR6 rapidly deactivate Gαo-GTP, so that the pool of Gαo-GTP declines with subsecond kinetics, and the cation channels open. Recently, Rao & Chen (2006) showed that Gβ5−/− mice completely lack the b-wave of the electroretinogram, which reflects the ON-bipolar cell response. These data suggest that the GTPase accelerating function of RGS7-Gβ5 and/or RGS11-Gβ5 is essential for ON-bipolar cell responses.
The presence of both Gβ5–RGS7 and Gβ5–RGS11 in the ON-bipolar cell dendrites raises the question of whether they are redundant complexes or whether they serve different functions in the ON-bipolar cell pathway. While both Gβ5–RGS7 and Gβ5–RGS11 have been shown to act as GAPs on Gαo, Gβ5–RGS11 has been shown to have twice the GAP activity of Gβ5–RGS7 (Hooks et al., 2003). The same study revealed that co-expression of Gβ5–RGS7 with Gβ5–RGS11 inhibited the GAP activity of Gβ5–RGS11 to levels closer to Gβ5–RGS7, suggesting interactions between the two complexes. The GAP activities of Gβ5–RGS7 and Gβ5–RGS11 are likely to be differentially regulated. RGS7, for example, has been shown to interact with an array of signaling molecules including R7BP (Martemyanov et al., 2005), 14-3-3, PKCα, phosphatidyl inositol-3, 4, 5-triphosphate (PIP3) and calmodulin (Benzing et al., 2002; Liu et al., 2005).
The molecular identity of the signaling cascade in ON-bipolar cells is a long-standing mystery in retinal physiology. The immunohisto-chemical evidence presented in this study suggests that Gβ5–RGS7 and Gβ5–RGS11 are likely to be key players in the cascade, and important determinants of the kinetics of the ON-bipolar cell light response.
Acknowledgements
We wish to thank Dr Larry Pinto, Northwestern University, for supplying the nob4 mouse strain, and Dr W. Rowland Taylor, OHSU, for critical reading of the manuscript. Grant support was received from NIH: S10 RR106858; and National Eye Institute: EY09534 (R.M.D.), 1R03EY016078 (C.W.M.), MH67094 (R.L.B.), EY11900 (T.G.W.).
Abbreviations
- BPC
bipolar cell
- GAP
GTPase-accelerating protein
- IPL
inner plexiform layer
- OPL
outer plexiform layer
- RGS
regulator of G-protein signaling
References
- Benzing T, Kottgen M, Johnson M, Schermer B, Zentgraf H, Walz G, Kim E. Interaction of 14–3–3 protein with regulator of g protein signaling 7 is dynamically regulated by tumor necrosis factor-alpha. J. Biol. Chem. 2002;277:32954–32962. doi: 10.1074/jbc.M200859200. [DOI] [PubMed] [Google Scholar]
- Berntson A, Taylor W. Response characteristics and receptive field widths of ON-bipolar cells in the mouse retina. J. Physiol. (Lond.) 2000;524:879–889. doi: 10.1111/j.1469-7793.2000.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Taylor WR, Morgans CW. Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. J. Neurosci. Res. 2003;71:146–151. doi: 10.1002/jnr.10459. [DOI] [PubMed] [Google Scholar]
- Berrebi AS, Oberdick J, Sangameswaran L, Christakos S, Morgan JI, Munaini E. Cerebellar purkinje cell markers are expressed in retinal bipolar neurons. J. Comp. Neurol. 1991;308:630–649. doi: 10.1002/cne.903080409. [DOI] [PubMed] [Google Scholar]
- Chen C-K, Burns ME, He W, Wensel TG, Baylor DA, Simon MI. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000;403:557–560. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- Chen C-K, Eversole-Cire P, Zhang H, Mancino V, Chen YJ, He W, Wensel TG, Simon MI. Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gbeta5. Proc. Natl Acad. Sci. USA. 2003;100:6604–6609. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Faurobert E, Dascal N, Sterling P, Vardi N. A retinal-specific regulator of g-protein signaling interacts with G{alpha}o and accelerates an expressed metabotropic glutamate receptor 6 cascade. J. Neurosci. 2004;24:5684–5693. doi: 10.1523/JNEUROSCI.0492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L, Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha (o) J. Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EJ, Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires Galphao. J. Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurobert E, Hurley JB. The core domain of a new retina specific RGS protein stimulates the GTPase activity of transducin in vitro. Proc. Natl Acad. Sci. USA. 1997;94:2945–2950. doi: 10.1073/pnas.94.7.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H. Immunocytochemical analysis of the mouse retina. J. Comp. Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- He W, Cowan CW, Wensel TG. RGS9, a GTPase accelerator for phototransduction. Neuron. 1998;20:95–102. doi: 10.1016/s0896-6273(00)80437-7. [DOI] [PubMed] [Google Scholar]
- Hooks SB, Waldo GL, Corbitt J, Bodor ET, Krumins AM, Harden TK. RGS6, RGS7, RGS9, and RGS11 stimulate GTPase activity of Gi family G-proteins with differential selectivity and maximal activity. J. Biol. Chem. 2003;278:10087–10093. doi: 10.1074/jbc.M211382200. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J. Comp. Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman A, Max M. Ggammal3 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nature Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Krispel CM, Chen C-K, Simon MI, Burns ME. Prolonged photoresponses and defective adaptation in rods of G {beta} 5−/− mice. J. Neurosci. 2003;23:6965–6971. doi: 10.1523/JNEUROSCI.23-18-06965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispel CM, Chen D, Melling N, Chen Y-J, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel T-G, Chen CK, Burns ME. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Lan K-L, Zhong H, Nanamori M, Neubig RR. Rapid kinetics of regulator of G-protein signaling (RGS) -mediated Galphai and Galphao deactivation. Galpha specificity of RGS4 and RGS7. J. Biol. Chem. 2000;275:33497–33503. doi: 10.1074/jbc.M005785200. [DOI] [PubMed] [Google Scholar]
- Liu W, Leitges M, Wang Q, Li Y, Wensel TG. Regulation of RGS7 function by calmodulin, PIP3, PKC, and neurogranin. Soc. Neurosci. Abstr. 2005:606.3. [Google Scholar]
- Martemyanov KA, Yoo PJ, Skiba NP, Arshavsky VY. R7 BP, a novel neuronal protein interacting with RGS proteins of the R7 family. J. Biol. Chem. 2005;280:5133–5136. doi: 10.1074/jbc.C400596200. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. Antibody to calretinin stains All amacrine cells in the rabbit retina: double-label and confocal analyses. J. Comp. Neurol. 1999;411:3–18. [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR. Photoreceptor calcium channels: Insight from night blindness. Vis. Neurosci. 2005;22:561–568. doi: 10.1017/S0952523805225038. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Ren G, Akileswaran L. Localization of nyctalopin in the mammalian retina. Eur. J. Neurosci. 2006;23:1163–1171. doi: 10.1111/j.1460-9568.2006.04647.x. [DOI] [PubMed] [Google Scholar]
- Nawy S. The metabotropic receptor mGluR6 may signal through G (o), but not phosphodiesterase, in retinal bipolar cells. J. Neurosci. 1999;19:2938–2944. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. cGMP-gated conductance in retinal bipoar cells is suppressed by the photoreceptor transmitter. Neuron. 1991;1:677–683. doi: 10.1016/0896-6273(91)90380-i. [DOI] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;11:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Vitaterna MH, Shimomura K, Siepka SM, McDearmon EL, Balannik V, Omura C, Lumayag S, Invergo BM, Glawe B, Cantrell DR, Inayat S, Olvera MA, Vessey KA, McCall MA, Maddox D, Morgans CW, Young B, Pletcher MT, Mullins RF, Troy JB, Takahashi JS. Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis. Neurosci. 2007;24:111–123. doi: 10.1017/S0952523807070149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov S, Yu K, Kozasa T, Wilkie TM. The regulators of G protein signaling (RGS) domains of RGS4, RGS10, and GAIP retain GTPase activating protein activity in vitro. Proc. Natl Acad. Sci. USA. 1997;94:7216–7220. doi: 10.1073/pnas.94.14.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AL, Chen C-K. Gβ5 is required for normal light response of ON-bipolar cells. IOVS 47. 2006 ARVO E-Abstract 5494. [Google Scholar]
- Schiells RA, Falk G. Glutamate receptors of the rod bipolar cell are linked to a cyclic GMP cascade via a G-protein. Proc. R. Soc. Lond. B. Biol. Sci. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Simonds WF, Zhang JH. New dimensions in G protein signalling: G beta 5 and the RGS proteins. Pharm. Ada Helv. 2000;74:333–336. doi: 10.1016/s0031-6865(99)00043-6. [DOI] [PubMed] [Google Scholar]
- Snow BE, Betts L, Mangion J, Sondek J, Siderovski DP. Fidelity of G protein beta-subunit association by the G protein gamma - subunit-like domains of RGS6, RGS7, and RGS11. Proc. Natl Acad. Sci. USA. 1999;96:6489–6494. doi: 10.1073/pnas.96.11.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Song H, Wensel TG, Sokolov M, Martemyanov KA. Localization and differential interaction of R7 RGS proteins with their membrane anchors R7 BP and R9AP in neurons of vertebrate retina. Mol. Cell. Neurosci. 2007;35:311–319. doi: 10.1016/j.mcn.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J. Comp. Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Watson AJ, Aragay AM, Slepak VZ, Simon MI. A novel form of the G protein beta subunit Gbeta 5 is specifically expressed in the vertebrate retina. J. Biol. Chem. 1996;271:28154–28160. doi: 10.1074/jbc.271.45.28154. [DOI] [PubMed] [Google Scholar]
- von Weizsacker E, Strathmann MP, Simon MI. Diversity among the beta subunits of heterotrimeric GTP-binding proteins: characterization of a novel beta-subunit cDNA. Biochem. Biophys. Res. Commun. 1992;183:350–356. doi: 10.1016/0006-291x(92)91650-f. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Wässle H. Responses of rod bipolar cells isolated from the rat retina to the glutamate agonist 2-amino-4-phosphonobutyric acid (APB) J. Neurosci. 1991;11:2372–2382. doi: 10.1523/JNEUROSCI.11-08-02372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Trexler EB, Massey SC. Confocal analysis of reciprocal feedback at rod bipolar terminals in the rabbit retina. J. Neurosci. 2002;22:10871–10882. doi: 10.1523/JNEUROSCI.22-24-10871.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wensel TG, Kraft TW. GTPase regulators and photoresponses in cones of the eastern chipmunk. J. Neurosci. 2003;23:1287–1297. doi: 10.1523/JNEUROSCI.23-04-01287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]