Abstract
Genetic data in the mouse have shown that endothelin 3 (ET3) and its receptor B (ETRB) are essential for the development of two neural crest (NC) derivatives, the melanocytes and the enteric nervous system. We report here the effects of ET3 in vitro on the differentiation of quail trunk NC cells (NCC) in mass and clonal cultures. Treatment with ET3 is highly mitogenic to the undifferentiated NCC population, which leads to expansion of the population of cells in the melanocytic, and to a lesser extent, the glial lineages. The effect of ET3 on these two NC derivatives was confirmed by the quantitative analysis of clones derived from individual NCC subjected to ET3: we found a large increase in the survival and proliferation of unipotent and bipotent precursors for glial cells and melanocytes, with no significant effect on multipotent cells generating neurons. ET3 first stimulates expression of both ETRB and ETRB2 by cultured NCC. Then, under prolonged exposure to ET3, ETRB expression decreases and switches toward an ETRB2-positive melanogenic cell population. We therefore propose that the present in vitro experiments (long-lasting exposure to a high concentration of ET3) mimic the environment encountered by NCC in vivo when they migrate to the skin under the ectoderm that expresses ET3.
Keywords: quail embryo/clonal cultures/endothelin receptors/in situ hybridization
The neural crest (NC) appears dorsally to the neural primordium as it forms according to a craniocaudal gradient. The presumptive NC cells (NCC) undergo an epithelio-mesenchymal transition and, after a phase of migration, give rise to multiple cell types including melanocytes, neurons, and glial cells of peripheral nerves and ganglia, a large majority of the cephalic mesenchymal structures, and certain endocrine cells (1, 2). Because of its pluripotentiality and the fact that its constitutive cells become localized in various regions of the developing embryo, the NC is an ideal developmental system in which to study the mode of action of factors involved in differentiation choices.
The importance of environmental influences on the development of NCC has been demonstrated by in vivo transplantation experiments in the chicken embryo and in vitro culture studies (1–6). At the onset of migration, the NCC population is composed of a mixture of pluripotent and more or less restricted progenitors that have been identified by various cell cloning experiments (7–14). These observations suggest that both selective and instructive mechanisms are involved in NCC diversification. Thus far, differentiation of definite lineages of NC-derived cells in clonal cultures has proved to be favored by factors such as brain-derived neurotrophic factor (15), glial growth factor (16), retinoic acid (17), and members of the transforming growth factor β family (18). Other growth factors (and their receptors) encoded by loci that affect NC derivatives in mice have been shown to have an important role in NC ontogeny but their mode of action is not yet fully understood. Such is the case for the receptor-ligand system constituted by endothelin receptor B (ETRB) and endothelin 3 (ET3). Mutations in the murine genes encoding ET3 and ETRB lead to an abnormal development of two NC derivatives: melanocytes and enteric ganglia, which are lacking in the terminal bowel (19–21). In the avian system we have identified two ETRB-like molecules, which are expressed by different populations of NCC during migration. ETRB is first expressed in the dorsal tip of the neural tube before emigration of NCC. It is later expressed by NCC migrating along the ventral migration route and, later in development, by adrenomedullary cells and all of the neural (peripheral and enteric) derivatives of the NC. Cells of the mesectodermal and melanocytic lineages do not express ETRB (22). An additional ETR has been isolated from avian pigment cells grown in culture, and designated ETRB2 (23), because of its close homology to ETRB. ETRB2 begins to be expressed by NCC migrating to the skin where they differentiate into melanocytes. Apart from a faint signal in dorsal root ganglia and nerves, ETRB2 expression by NC derivatives is almost completely restricted to pigment cells and their precursors (23). We previously have shown that ET3 strongly promotes NCC proliferation, eventually resulting in a large increase in the number of melanocytes developing in quail NCC cultures (24). Here we further analyze the effects of ET3 on NCC, by using culture conditions that permit all of the phenotypes of cells derived from trunk NC precursors to develop (8, 9, 11, 17). We particularly scrutinize the role played by ET3 in the expression of ETRB and ETRB2 by quail NCC during the culture and in the phenotypic choices made by individual cells in clonal cultures. We find that ET3 selectively promotes the survival and differentiation of two types of NC progenitors: those that give rise to melanocytes and glia. Moreover we also demonstrate that, in culture, NCC first express ETRB and then, in response to ET3, switch to ETRB2.
MATERIALS AND METHODS
Secondary and Clonal Cultures of Trunk NCC.
NCC were obtained from explants of trunk neural tubes cultured for 20 h as described (17, 25). Growth-arrested Swiss 3T3 fibroblasts provided a substrate on which to subculture NCC (8). Secondary cultures were performed by seeding 5 × 103 cells in 30-μl control culture medium on established feeder-layers prepared the day before on plastic culture dishes (Costar). The procedure for preparing clonal cultures of NCC was essentially as described (8, 9, 11, 17). Single cells were picked up from the crest cell suspension under microscopic control and were individually seeded onto 3T3-cell feeder-layers by means of a micropipette (25, 26).
After 3 h, NCC had attached and cultures were fed with control medium ± ET3 (rat, human; Sigma) at a concentration of 100 nM, a dose previously shown to optimally increase proliferation of quail NCC (24). Control medium consisted of Brazeau Basic Medium (27) supplemented with 10% fetal calf serum (GIBCO/BRL) and 2% chicken embryo extract. Medium was changed on day 3 (d3) and d6. The cultures were maintained at 37°C in an atmosphere of 5% CO2 and 95% air and were analyzed at d9.
Immunocytochemistry and Clone Analysis.
To assess the presence of differentiated cell types, NCC cultures were analyzed by immunocytochemistry with several phenotypic markers. Glial cells were identified by using Schwann cell myelin protein (SMP) mAb (11, 28). Neurons were recognized with a rabbit antiserum against 200-kDa neurofilament protein (Sigma) and antityrosine hydroxylase mAb was used to identify adrenergic cells (17). Cells differentiating along the melanocytic lineage were characterized by using the melanoblast/cyte early marker mAb, which labels NC-derived pigment cells and melanoblasts before pigmentation starts (29). Melanocytes also were detected microscopically by the presence of pigment granules. Immunocytochemistry was carried out as described (17). In some experiments, SMP immunoreactivity was revealed by using the direct tyramide signal amplification kit (NEN) according to the manufacturer’s instructions. Fluorescence was observed with a UV-illuminated Zeiss Orthoplan microscope.
Single-cell cultures of trunk NCC were analyzed as described (11, 17). The clones were detected after staining with the nuclear dye bisbenzimide (Serva) (8) and labeled with the anti-SMP mAb. Cells then were permeabilized, and antibodies to neurofilament protein and antityrosine hydroxylase were applied together. Finally, after documenting glial cells and neurons, cultures were labeled with the melanoblast/cyte early marker mAb, and melanocytes were counted. To quantify cell proliferation in clonal cultures, the total number of cells was evaluated at d9: in controls, the total number of quail cell nuclei was counted after bisbenzimide staining; in ET3-treated cultures, the higher rate of cell growth precluded such counting and the total cell number was determined by counting nuclei on 10 randomly selected microscopic fields. Colonies containing more than 1 × 103 cells were defined as large clones. The frequencies of the various types of clones were analyzed by the χ2 test (Statistica for Macintosh, Statsoft), and differences between values from ET3-treated and control cultures were considered to be statistically significant when P < 0.05.
In Situ Hybridization on NCC Cultures.
The expression of ET3 receptors by NCC in vitro was studied by in situ hybridization on secondary cultures of NCC at different time points of the culture period. Sense and antisense riboprobes for avian ETRB and ETRB2 were transcribed and digoxigenin (dig)-labeled by using dig-11-UTP (Boehringer Mannheim) (22, 23). In situ hybridization was performed at high stringency according to Henrique et al. (30). The hybridized probes were demonstrated according to standard procedure using an alcaline phosphatase-conjugated antidig antibody (Boehringer Mannheim), followed by incubation with color substrate solution (30). Staining was visualized and photographed with an inverted Olympus CK2 microscope. No hybridization signal was obtained with control sense probes on NCC cultures either with or without ET3. No hybridization was observed with feeder layers of mouse 3T3 cells cultured alone, with, or without ET3 (not shown).
To investigate whether individual NCC express both ETRB and ETRB2, double in situ hybridization experiments were performed on NCC cultured in control medium on collagen-coated 8-microwell LabTeck glass slides (Nunc), in the absence of a 3T3 feeder layer. Cultures were fixed on d3 as described above and incubated overnight at 52°C in hybridization buffer (31) containing both the 35S-labeled ETRB (22) and the dig-labeled ETRB2 riboprobes. Washing was carried out at 65°C in 50% formamide before and after RNase treatment. The ETRB2 labeling was revealed as described above before ETRB autoradiography (22). Epipolarization filters allowed simultaneous visualization of both signals (32).
RESULTS
ET3 Promotes the Expansion of ETRB- and ETRB2-Expressing Cells in NCC Cultures.
Trunk NCC were allowed to migrate in vitro from isolated neural tubes for 20 h and then replated on a 3T3 fibroblast-feeder layer. These culture conditions were shown to permit NCC to differentiate along their major lineages (refs. 9 and 17; see also next section). The expression by NCC of ETRB and ETRB2 genes was assessed by in situ hybridization with dig-labeled riboprobes. ET3-treated and control cultures were compared at different time points. After d3 of secondary culture in control medium, only a few ETRB2-labeled crest cells were seen at the border of the cultured 3T3 cells (Fig. 1A). ETRB-expressing cells, on the other hand, were found dispersed on the 3T3 cells (Fig. 1C). These ETRB-positive NCC were slightly more numerous but showed a lower expression level (as judged by the lower labeling intensity) than those expressing ETRB2.
Figure 1.
Expression of ETRB2 and ETRB by quail NCC cultured in the absence (Left) and presence (Right) of ET3. In situ hybridization with ETRB2 (A, B, E, and F) and ETRB (C, D, G, and H) dig-labeled riboprobes. (A–D) At d3 ET3 promotes increase in ETRB2 (A and B) and ETRB (C and D) transcripts. (E–H) At d6, ET3-treated cultures show enhanced expression of ETRB2 (compare F to E: in F, the positive cells in blue occupy most of the microscopic field) and down-regulation of ETRB (G and H). (Bar = 90 μm.)
In the presence of ET3 at the same time point (d3) a large increase in the number of cells expressing either receptor was seen (Fig. 1 B and D). Cells expressing ETRB2 were widespread all over the cultures (Fig. 1B) and not localized preferentially to the periphery, as they were in ET3-deprived cultures. Later on, expression of the two receptors differed. In d6 cultures treated with ET3 the population of ETRB2-positive cells was greatly increased (Fig. 1F) over that found in control cultures (Fig. 1E), whereas ETRB labeling decreased significantly (Fig. 1 G and H).
At d9, in the presence of ET3, the entire culture appeared black, because of the massive differentiation of melanocytes (Fig. 2A). Pigment cells were ETRB2-positive as clearly could be seen in regions of the cultures with a relatively low cell density (Fig. 2B).
Figure 2.
Melanogenesis and ETRB2 expression in d9-NCC cultures. (A) View of the culture dishes showing increased pigmentation of ET3-treated (Upper), as compared with control (Lower) cultures. (B) In situ hybridization of control cultures shows that pigment cells (with brown melanin) express ETRB2 (blue staining). (Bar = 40 μm.)
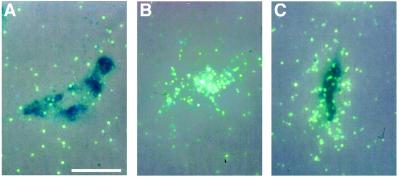
Because numerous ETRB- and ETRB2-positive cells were detected on d3, we investigated whether single NCC might coexpress the two receptors. For this purpose, double in situ hybridization with radioactive- and dig-labeled probes was performed on NCC cultured for 3 d in control medium in absence of 3T3 fibroblasts. Most of the cells were found to express either ETRB or ETRB2, whereas some cells were negative for both probes; in addition, a few individual cells expressed both ETRB and ETRB2 (Fig. 3), displaying moderate expression levels of the two genes. A possible interpretation is that these cells only transiently coexpress the two receptors, as they switch from one type (ETRB) of receptor to the other (ETRB2).
Figure 3.
Expression of ETRB and ETRB2 by individual NCC after double in situ hybridization. D3-cultures hybridized with both radioactive ETRB and dig-labeled ETRB2 probes are viewed under epipolarized illumination. Individual cells express either ETRB2 (A) or ETRB (B) alone, or both (C), as detected by the presence of antidig staining and silver grains. (Bar = 16 μm.)
In a previous work, we showed that ET3 massively increases the rate of proliferation of cultured NCC and also promotes melanogenic differentiation (24). We show here that ET3 initially causes expansion of both ETRB- and ETRB2-bearing NCC populations, ET3 then promotes expression only of ETRB2, which correlates with melanogenic differentiation. This finding explains why the prominent effect of ET3 in vitro is to promote growth and differentiation of melanogenic NCC. The initial expansion of the ETRB-positive cell population in NCC cultures suggests that pluripotent cells, including those that give rise to neural derivatives respond to ET3. We therefore examined the differentiation of neurons and glial cells in these cultures in which the predominent phenotype was melanocytic.
ET3 Induces the Expansion of the Glial Cell Lineage Together with That of Melanocytes.
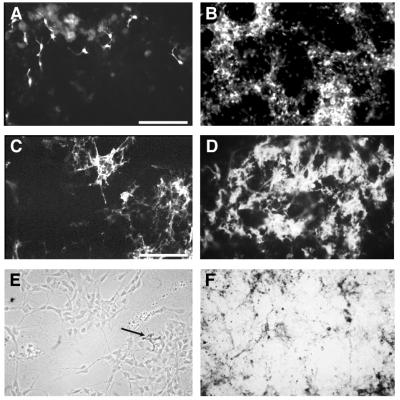
Immunofluorescence labeling with the various mAbs (see Material and Methods) was evaluated only by microscopic inspection, because cell counting was precluded by the high cell density found in ET3-treated cultures. Nevertheless, in 10 different experiments, the production of the diverse cell types consistently showed striking differences between control and ET3-treated cultures. In d9-control cultures, very few melanoblast/cyte early marker- positive cells appeared located at the periphery of the cultures (Fig. 4A), whereas in the presence of ET3, labeled pigment cell aggregates were recorded all over the cultures (Fig. 4B). Similarly, many more SMP+ cells were observed in ET3-treated than in control d9-cultures (Fig. 4 C–F). The number of cells belonging to glial and melanocytic lineages already was increased in d3-cultures in the presence of ET3 (not shown). In contrast, addition of ET3 caused no significant change in neuronal NCC differentiation at d9 because antityrosine hydroxylase-positive and neurofilament protein-positive cells were present in similar numbers in control and treated cultures (data not shown).
Figure 4.
Differentiation of melanoblasts and glial cells in d9-NCC cultures in the absence (Left) and presence (Right) of ET3. (A and B) The population of melanoblast/cyte early marker-positive melanoblasts increases after ET3 treatment (B) as compared with controls (A). (C–F) SMP-immunoreative glial cells also expand in response to ET3 (compare C and D); pigment cells are visible in E (arrow) and F (same fields as C and D, respectively). A–D, UV light; E, phase-contrast; F, bright-field. (Bars = 260 μm in A and B; 83 μm in C–F.)
It thus appears so far that ET3 is a potent mitogen for NCC and mostly promotes the growth of glial cells and melanocytes. It was therefore of interest to determine the nature of the precursors that respond to ET3. We thus analyzed NCC clonal cultures to investigate how ET3 affects pluripotent and/or already determined precursors of these cell lineages.
ET3 Promotes the Survival and Proliferation of a Bipotent Glia-Melanocyte Precursor and Unipotent Precursors for Glia and Melanocytes.
Clonal cultures were grown in control medium alone or after the addition of 100 nM ET3 and analyzed 9 d later. For each clone, the total number of cells per clone and the different cell phenotypes were evaluated. The results from four different cloning experiments, with a total of 525 plated single cells are as follows: first, ET3 had a striking effect on the cloning efficiency, which was 24.6% in control medium and 61.1% in the presence of ET3 (Table 1). Therefore, the capacity of isolated NCC to generate a clone and their survival rate for 9 d are increased 2.5-fold in the presence of ET3. The clones were classified into six categories according to the combination of the phenotypes they contained. Then, the frequency of each clone, expressed in percent of cells initially plated, was established and compared in control and ET3-supplemented cultures. ET3 promoted the survival and growth of three types of precursors: those generating both melanocytes and glial cells (i.e., Glia-Mel), those generating glial cells only (i.e., Glia), and melanocytes only (i.e., Mel) (Table 1). Upon addition of ET3, the frequency of unipotent glial precursors increased from 9.1% to 19.4% of plated cells (×2.1), whereas the frequencies of Mel and Glia-Mel precursors increased 16- and 13.4-fold, respectively (Table 1). In contrast, no significant ET3-induced change was found in the frequency of the other precursors: those yielding both neurons and glial cells (i.e., Glia-Neuro); those yielding neurons, glial cells and melanocytes (i.e., Glia-Neuro-Mel), and those giving rise to cells in which none of the markers tested was represented (i.e., no marker; Table 1).
Table 1.
Clonal efficiency and phenotypic analysis of NC precursors
| Precursor type | Clonal efficiency, % of clones from total plated cells
|
||
|---|---|---|---|
| Control | ET3 | ET3/control | |
| Mel | 0.57 | 9.1 | ×16 |
| Glia-Mel | 1.4 | 18.8 | ×13.4 |
| Glia | 9.1 | 19.4 | ×2.1 |
| Glia-Neuro | 5.4 | 5.7 | n.s. |
| Glia-Mel-Neuro | 1.4 | 0.5 | n.s. |
| No marker | 6.5 | 7.4 | n.s. |
| Total | 24.6 | 61.1 | ×2.5 |
The clonal efficiency of NCC, defined by the percentage of total plated cells that give rise to a colony, was calculated in d9-clonal cultures grown in the absence (control) and in the presence of 100 nM ET3 (ET3). Precursor types were categorized according to cell phenotypes in their progeny (see Materials and Methods). Values were obtained from four different experiments containing 350 control and 175 treated cultures. Differences between treated and control values are expressed as the ratio of ET3 over control values (ET3/control). Only those that were statistically significant (P < 0.01) are indicated here. n.s., not significant.
The other dramatic effect of ET3 was on the proliferative potential of clonogenic cells: in control cultures in most cases the total number of cells per clone was approximately 200 cells, whereas it could reach several thousand cells in ET3-treated cultures. Quantitation of large clones (defined here as those containing more than 1 × 103 cells) under both conditions showed that they accounted for 10.5% of control colonies. This proportion increased to more than 60% in ET3-treated clones (Table 2). The increase in the number of cells per clone was particularly remarkable in colonies derived from melanocyte and glial precursors. The proportion of large clones containing only glial cells was raised 52.8% in the presence of ET3 as compared with control medium, indicating that ET3 exerts a mitogenic effect on committed glial precursors. ET3 also promoted proliferation of melanogenic unipotent or bipotent precursors: whereas in control medium none of these precursors yielded a large progeny, in the presence of ET3, 75% of melanocyte clones and 70% of Glia-melanocyte clones were of large size (Table 2). Most of the colonies generated from multipotent neuronal progenitors were larger than 1 × 103 cells in either control or ET3-supplemented conditions. ET3 also increased the proportion of large colonies formed by cells bearing none of the markers tested (Table 2). In summary, analysis of single-cell cultures of NCC shows that ET3 selectively enhances the survival and proliferation of bipotent and unipotent precursors for glial cells and melanocytes.
Table 2.
Frequency of large colonies derived from NCC
| Precursor type | Frequency of large colonies (% of large clones from total clones)
|
||
|---|---|---|---|
| Control | ET3 | Δ% | |
| Glia | 1/32 (3.1) | 19/34 (55.9) | +52.8* |
| Glia-Mel + Mel | 0/7 (0) | 35/49 (71.4) | +71.4* |
| Glia-Neuro + | |||
| Glia-Mel-Neuro | 8/24 (33) | 8/11 (72.7) | +39.7 |
| No marker | 0/23 (0) | 3/13 (23) | +23* |
| Total | 9/86 (10.5) | 65/107 (60.7) | +50.2* |
A total of 193 clones (86 in control medium and 107 in the presence of ET3, same clones as in Table 1) was examined at d9 for the total cell number and cell phenotypes. Clones containing more than 1 × 103 cells were categorized as large clones. Values are expressed as the proportion and percent (%) of large clones from the total number of clones generated by each precursor type. Differences between ET3 and control values (Δ%) are indicated by ∗ when statistically significant (P < 0.05).
DISCUSSION
The alteration of pigment pattern (color spotting) seen in mice carrying spontaneous or targeted mutations in the genes encoding ET3 and ETRB (19–21) has prompted recent interest in the role played by this signaling pathway in the differentiation of melanocytes from the NC. In a previous in vitro study, we have shown that ET3 is a very potent mitogen for NCC because a large increase in cell proliferation, and later on in the number of differentiated melanocytes in quail NCC cultures, was observed upon addition of ET3 to culture media (24). Endothelins also promote melanogenesis in mouse NCC cultures (33). These observations suggest that ET3 may act by triggering the expansion of the melanocytic lineage. The possibility also has been raised that ET3 favors the development of melanocytes at the expense of the other NC-derived (e.g., glial and neuronal) lineages, thus acting in an instructive manner. To address these questions, we have, in the present work, further examined the effect of ET3 on the differentiation of quail NCC into its various derivatives.
When NCC leave the neural fold in avian embryos, virtually all NC-derived cells express ETRB (22). Recently a receptor called ETRB2 has been identified (23) and shown to be expressed by the melanoblasts and melanocytes that migrate to the skin. One characteristic of the migration of the avian trunk NCC is that they take the dorsoventral migration route, leading them to form sensory and sympathetic ganglia first, at a time when the mediolateral pathway that will be taken by the future melanocytes en route to the skin is not yet accessible. Thus, NCC wait for 24–36 h in the so-called staging area (34) before they can take this mediolateral route. Interestingly, in the avian embryo in vivo, two phases can be distinguished, an early one, where virtually all NCC express ETRB, including those that will later become melanocytes; and a second phase, when NCC migrate laterally underneath the epidermis. Those cells lose the expression of ETRB and acquire that of ETRB2. It was therefore of interest to study the dynamics of expression of ETRB and ETRB2 by NCC in vitro.
A Prolonged Effect of ET3 in Culture Promotes the Switch from ETRB to ETRB2 Expression by NCC.
The optimal dose of ET3 of 100 nM based on our previous results (24) was used throughout this investigation. At that concentration, and after d1 and d3 of secondary culture, ET3 had considerably increased the proliferation rate of NCC. We now find that, after d3 of secondary culture, the majority of NCC express ETRB, while, at the same time, a large number of cells also express ETRB2. In addition, we show by double in situ hybridization that coexpression of the two receptors can occur in individual cells. After 6 d of treatment with ET3, ETRB2 is expressed by most cells, whereas only a few can be detected with small amounts of ETRB mRNA. It therefore can be concluded that both ETRB and ETRB2 mediate the early proliferative response of NCC in culture. From d5 of culture in the presence of ET3, however, the proliferation rate plateaus and later decreases. When this process commences, the cells begin to express melanocytic markers (24). One therefore can assume that this second effect of ET3 on NCC, characterized by the recruitment of a large number of cells to the melanocytic differentiation pathway, is mediated by ETRB2, the expression of which is not only maintained, but increased in the cultures subjected to a prolonged exposure to ET3. At the same time, expression of ETRB is down-regulated. These observations support the idea that ET3 induces (or promotes) the expression of ETRB2 under these conditions.
The in vitro responses to massive and prolonged exposure of NCC to ET3 provide insight into the in vivo observations. As they leave the neural primordium and spread throughout the embryo, the NCC significantly increase in number. Our in vitro results suggest that ET3 may play a role in this process via ETRB. When, at embryonic d3, the mediolateral pathway becomes available for NCC migration, the superficial ectoderm strongly expresses the ET3 gene (35) and therefore ET3 may, at this time, be produced at concentrations high enough to induce expression of ETRB2. When ETRB2 is induced, ETRB gene activity is down-regulated, and the cells that have taken the mediolateral migration route develop as melanocytes. Our previous in vivo observations have shown that the cells located in the subectodermal mesenchyme express ETRB2, and not ETRB (22, 23). We therefore propose that high concentrations of ET3 favor the switch from ETRB to ETRB2 and that coexpression of the two receptors by the same cells is probably only a transient phenomenon resulting from culture conditions and may not occur in vivo.
The next step in our study was to determine whether ET3 has a specific effect on certain NC precursors and, if so, on which ones. We tested the relative influence of ET3 on survival, proliferation, and differentiation of identified NC progenitors by using a clonal culture assay that was developed in our laboratory (8, 9, 11, 17).
ET3 Has a Selectively Positive Effect on Survival and Proliferation of Melanogenic and Glial but Not Neuronal Progenitors.
The analysis of the progeny of individual NCC in culture reported here first shows that ET3 strongly enhances the ability of NC precursor cells to survive and proliferate. Although six types of clonogenic precursors were recorded in the absence or presence of ET3, they did not respond equally to ET3. The frequency of the various clonal types revealed that only three types of NCC, i.e., the glial and melanocytic unipotent and bipotent precursors, are targets for the survival- and growth-promoting activity of ET3. This activity is weaker on the glial than the melanocytic precursors, which are rescued by ET3. These effects are therefore likely to be responsible for the large increase in melanocytes and glial cells that also was observed in mass cultures of NCC.
Our present study shows that ET3 does not significantly influence the development of multipotent neuronal progenitors. This observation contrasts with a recent finding that pluripotent quail NCC with neuronal potentialities respond to ET3 in vitro (36). The different experimental designs used in the two studies may explain these discrepancies. The conclusion by Stone et al. (36) was drawn from analyses of the phenotypes of cells derived from NCC that were first cultured during a short period with ET3 and then left to differentiate without it. In the present study, NCC were submitted to long-term treatment with ET3 (during the whole culture period). Consequently, under the conditions used in the present study, neuronal precursors, which stop dividing and differentiate early, may have responded transiently to ET3 during the early period of culture. If exposure to ET3 is prolonged, the development of differentiated neurons is not stimulated. Indeed, we have detected a promoting effect on neuronal precursors, after a transient treatment with ET3 (unpublished results), meaning that pluripotent NCC respond to the proliferative effect of ET3.
ET3 also enhances the proliferation of NCC that generate cells expressing none of the lineage markers tested here. Such cells, not reactive for melanocytic or neural markers and of fibroblastic-like morphology, previously have been observed under various culture conditions (8, 11, 36, 37). It is not presently known whether these cells are precursors that remain undifferentiated in vitro or whether they differentiate later. Alternatively they may express differentiation markers not tested here and thus represent other cell types.
No significant decrease was observed in the number of neuronal or undefined clonal types, suggesting that ET3 does not bias NCC differentiation toward melanocytes at the expense of nonmelanocytic cell types. It thus seems unlikely that ET3 modifies the choice of NCC phenotypes in an instructive manner. Instead, data suggest a selective action of ET3, which consists of promoting the survival of melanogenic precursors that would have died in the absence of ET3. We have shown recently that ET3 is expressed in the dorsal ectoderm when avian NCC migrate (35). Thus, NCC that migrate dorsoventrally are exposed only transiently to ET3, whereas NCC that migrate along the mediolateral subectodermal pathway encounter high concentrations of ET3 during the whole period of skin colonization. Our in vitro cloning conditions, which promote the development of melanogenic precursors, therefore mimic this mediolateral environment. In contrast, a transient exposure of cultured NCC to ET3 is closer to the dorsoventral environment of neurogenic precursors.
Acknowledgments
We thank F. Beaujean and F. Viala for the illustrations and C. Guilloteau for help in preparing the manuscript. This work was supported by the Centre National de la Recherche Scientifique and by grants from the Association pour la Recherche contre le Cancer and Ligue Nationale contre le Cancer.
ABBREVIATIONS
- ET3
endothelin 3
- ETRB
endothelin receptor B
- NC
neural crest
- NCC
neural crest cells
- d
day
- SMP
Schwann cell myelin protein
- dig
digoxigenin
References
- 1.Le Douarin N. The Neural Crest. Cambridge, U.K.: Cambridge Univ. Press; 1982. [Google Scholar]
- 2.Le Douarin N M, Dupin E, Ziller C. Curr Opin Genet Dev. 1994;4:685–695. doi: 10.1016/0959-437x(94)90135-p. [DOI] [PubMed] [Google Scholar]
- 3.Bronner-Fraser M. Curr Opin Genet Dev. 1993;3:641–647. doi: 10.1016/0959-437x(93)90101-t. [DOI] [PubMed] [Google Scholar]
- 4.Stemple D L, Anderson D J. Dev Biol. 1993;159:12–23. doi: 10.1006/dbio.1993.1218. [DOI] [PubMed] [Google Scholar]
- 5.Sieber-Blum M, Ito K, Richardson M K, Langtimm C J, Duff R S. J Neurobiol. 1993;24:173–184. doi: 10.1002/neu.480240205. [DOI] [PubMed] [Google Scholar]
- 6.Le Douarin N M, Kalcheim C. The Neural Crest. 2nd Ed. Cambridge: Cambridge Univ. Press; 1999. , in press. [Google Scholar]
- 7.Sieber-Blum M, Cohen A M. Dev Biol. 1980;80:96–106. doi: 10.1016/0012-1606(80)90501-1. [DOI] [PubMed] [Google Scholar]
- 8.Baroffio A, Dupin E, Le Douarin N M. Proc Natl Acad Sci USA. 1988;85:5325–5329. doi: 10.1073/pnas.85.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baroffio A, Dupin E, Le Douarin N M. Development (Cambridge, UK) 1991;112:301–305. doi: 10.1242/dev.112.1.301. [DOI] [PubMed] [Google Scholar]
- 10.Sieber-Blum M. Dev Biol. 1989;136:372–380. doi: 10.1016/0012-1606(89)90263-7. [DOI] [PubMed] [Google Scholar]
- 11.Dupin E, Baroffio A, Dulac C, Cameron-Curry P, Le Douarin N M. Proc Natl Acad Sci USA. 1990;87:1119–1123. doi: 10.1073/pnas.87.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser S E, Bronner-Fraser M. Development (Cambridge, UK) 1991;112:913–920. doi: 10.1242/dev.112.4.913. [DOI] [PubMed] [Google Scholar]
- 13.Stemple D L, Anderson D J. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 14.Henion P D, Weston J A. Development (Cambridge, UK) 1997;124:4351–4359. doi: 10.1242/dev.124.21.4351. [DOI] [PubMed] [Google Scholar]
- 15.Sieber-Blum M. Neuron. 1991;6:949–955. doi: 10.1016/0896-6273(91)90235-r. [DOI] [PubMed] [Google Scholar]
- 16.Shah N M, Marchionni M A, Isaacs I, Stroobant P, Anderson D J. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 17.Dupin E, Le Douarin N M. Dev Biol. 1995;168:529–548. doi: 10.1006/dbio.1995.1100. [DOI] [PubMed] [Google Scholar]
- 18.Shah N M, Groves A K, Anderson D J. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 19.Hosoda K, Hammer R E, Richardson J A, Baynash A G, Cheung J C, Giaid A, Yanagisawa M. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 20.Puffenberger E G, Hosoda K, Washington S S, Nakao K, Dewit D, Yanagisawa M, Chakravarti A. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 21.Baynash A G, Hosoda K, Giaid A, Richardson J A, Emoto N, Hammer R E, Yanagisawa M. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 22.Nataf V, Lecoin L, Eichmann A, Le Douarin N M. Proc Natl Acad Sci USA. 1996;93:9645–9650. doi: 10.1073/pnas.93.18.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lecoin L, Sakurai T, Ngo M T, Abe Y, Yanagisawa M, Le Douarin N M. Proc Natl Acad Sci USA. 1998;95:3024–3029. doi: 10.1073/pnas.95.6.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahav R, Ziller C, Dupin E, Le Douarin N M. Proc Natl Acad Sci USA. 1996;93:3892–3897. doi: 10.1073/pnas.93.9.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupin E, Le Douarin N M. In: Essential Developmental Biology: A Practical Approach. Stern C D, Holland P W H, editors. New York: Oxford Univ. Press; 1993. pp. 153–166. [Google Scholar]
- 26.Dupin E, Ziller C, Le Douarin N M. In: Cellular and Molecular Procedures in Developmental Biology. de Pablo F, Ferrus A, Stern C D, editors. San Diego: Academic; 1998. pp. 1–35. [Google Scholar]
- 27.Ziller C, Dupin E, Brazeau P, Paulin D, Le Douarin N M. Cell. 1983;32:627–638. doi: 10.1016/0092-8674(83)90482-8. [DOI] [PubMed] [Google Scholar]
- 28.Dulac C, Cameron-Curry P, Ziller C, Le Douarin N M. Neuron. 1988;1:211–220. doi: 10.1016/0896-6273(88)90141-9. [DOI] [PubMed] [Google Scholar]
- 29.Nataf V, Mercier P, Ziller C, Le Douarin N M. Exp Cell Res. 1993;207:171–182. doi: 10.1006/excr.1993.1177. [DOI] [PubMed] [Google Scholar]
- 30.Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Nature (London) 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- 31.Wakamatsu Y, Kondoh H. Acta Histochem Cytochem. 1990;23:367–374. [Google Scholar]
- 32.Lecoin L, Lahav R, Martin F H, Teillet M A, Le Douarin N M. Dev Dyn. 1995;203:106–118. doi: 10.1002/aja.1002030111. [DOI] [PubMed] [Google Scholar]
- 33.Reid K, Turnley A M, Maxwell G D, Kurihara Y, Kurihara H, Bartlett P F, Murphy M. Development (Cambridge, UK) 1996;122:3911–3919. doi: 10.1242/dev.122.12.3911. [DOI] [PubMed] [Google Scholar]
- 34.Wehrle-Haller B, Weston J A. Development (Cambridge, UK) 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- 35.Nataf V, Amamiya A, Yanagisawa M, Le Douarin N M. Mech Dev. 1998;73:217–220. doi: 10.1016/s0925-4773(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 36.Stone J G, Spirling L I, Richardson M K. J Cell Sci. 1997;110:1673–1682. doi: 10.1242/jcs.110.14.1673. [DOI] [PubMed] [Google Scholar]
- 37.Rogers S L, Gegick P J, Alexander S M, McGuire P G. Dev Biol. 1992;151:192–203. doi: 10.1016/0012-1606(92)90226-7. [DOI] [PubMed] [Google Scholar]