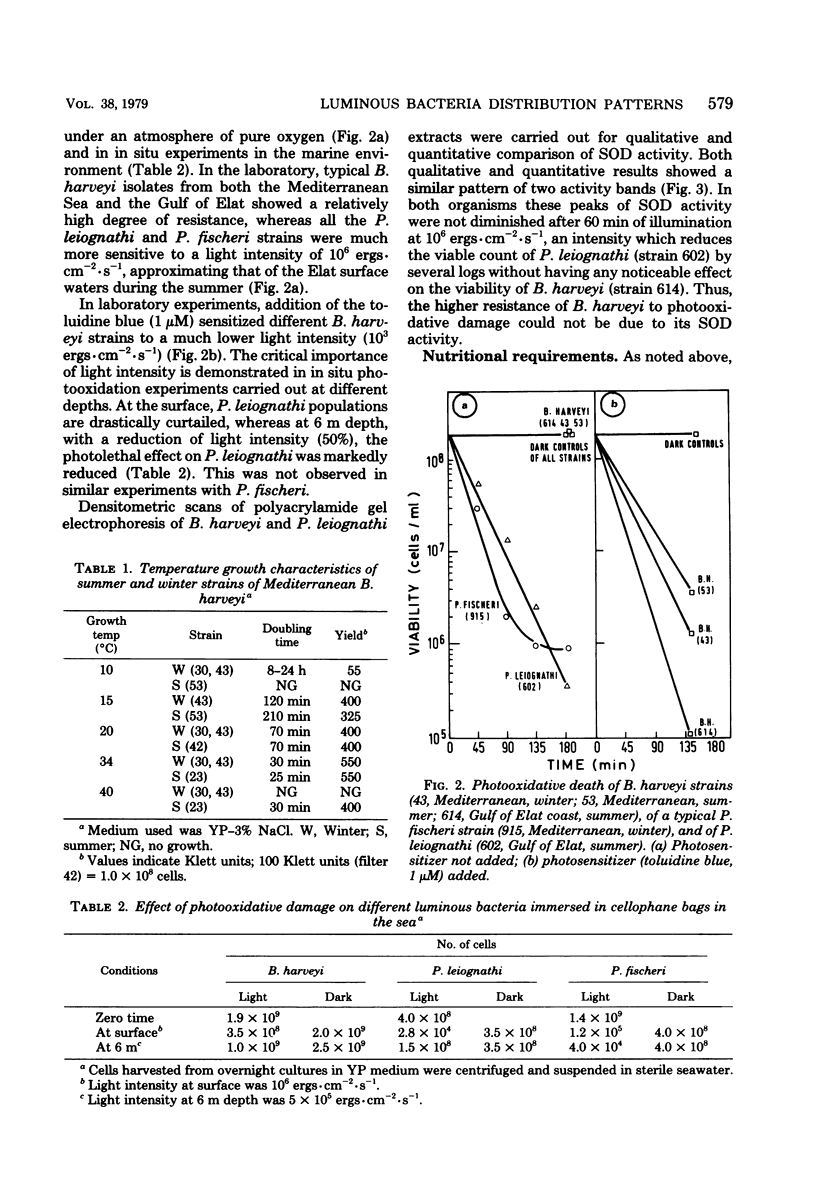
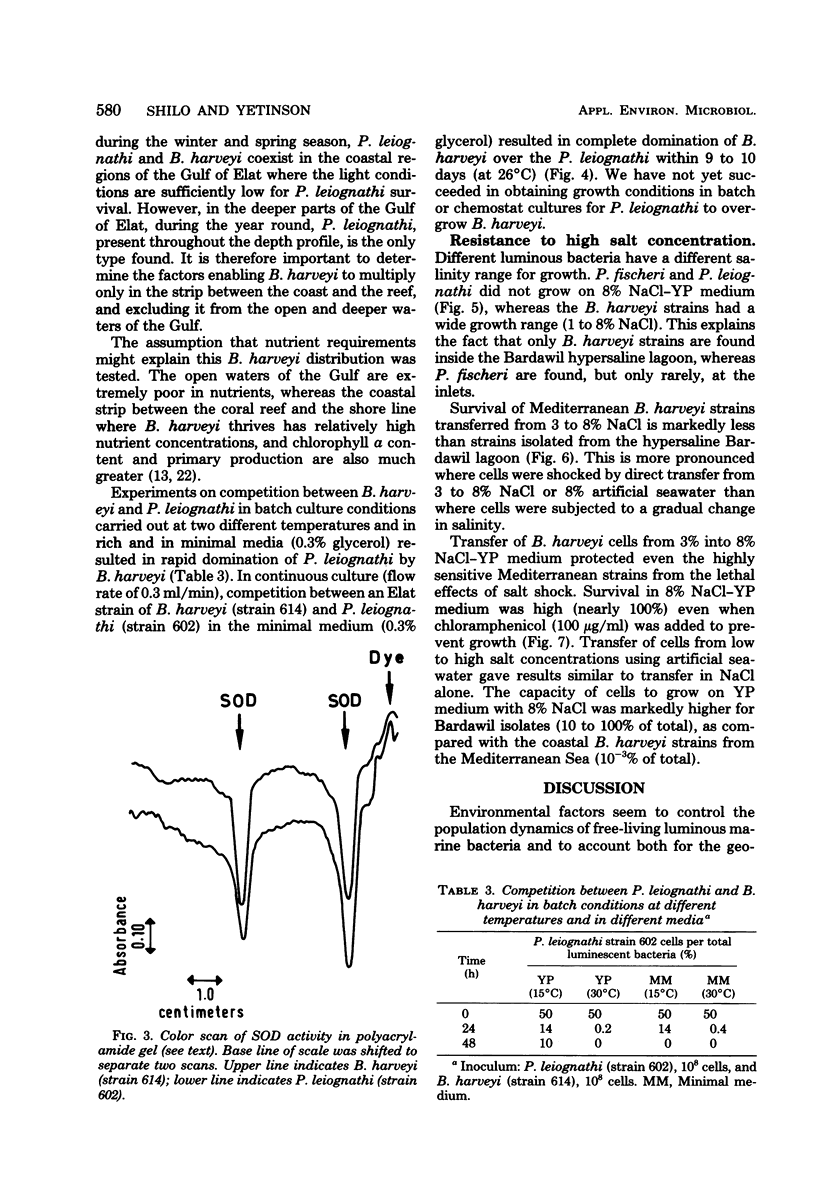
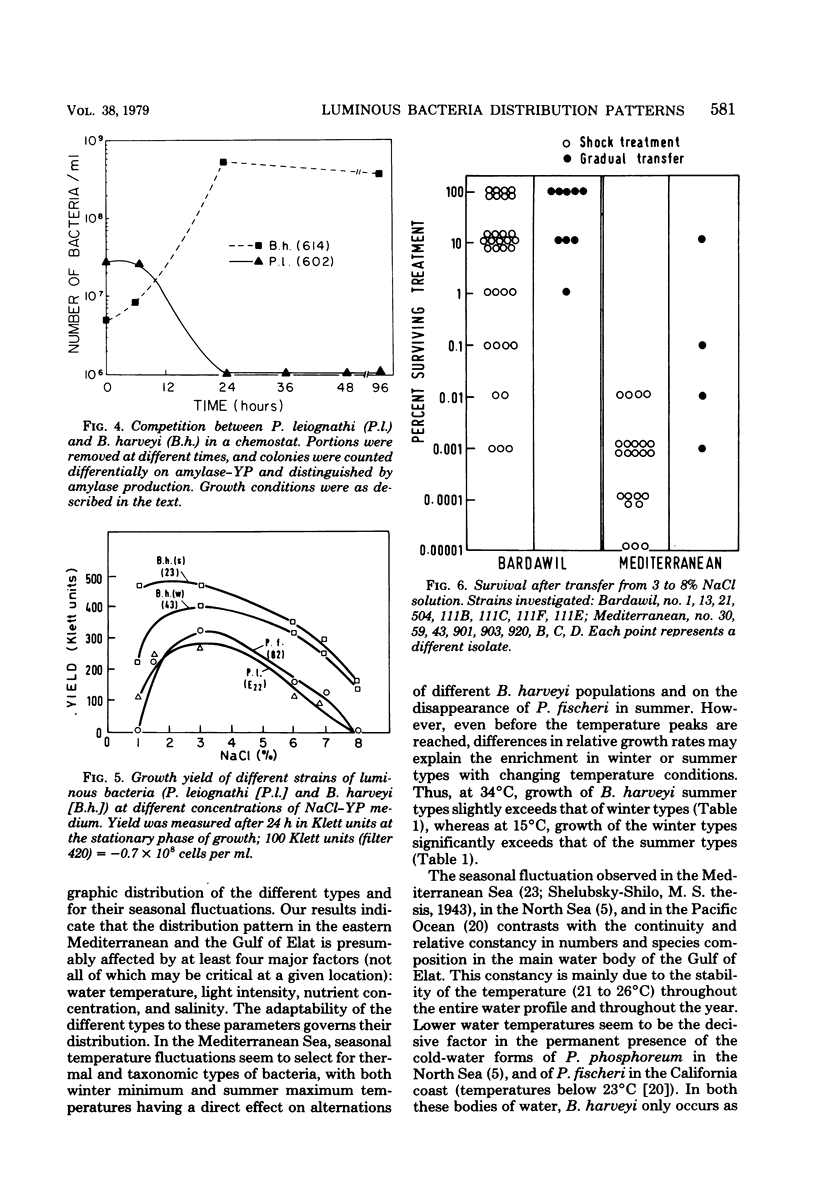
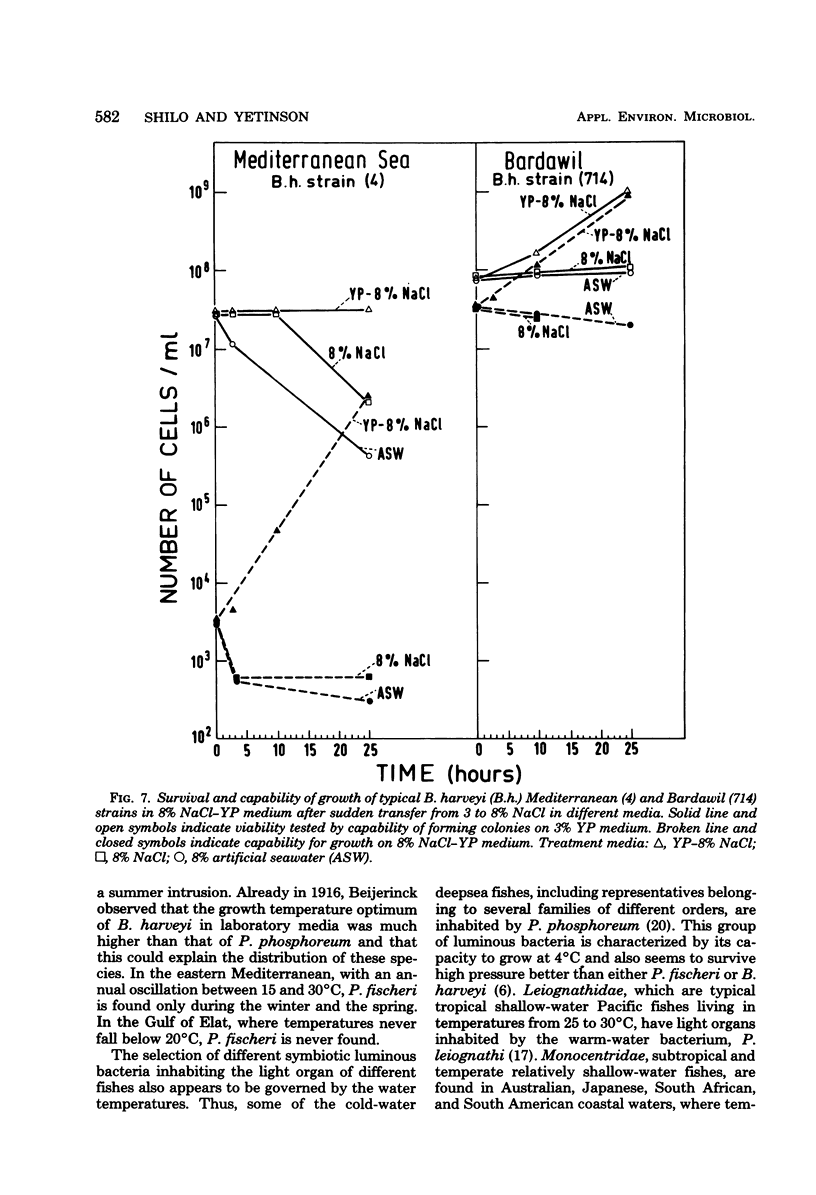
Abstract
Physiological characteristics of luminous bacteria isolated from the Mediterranean and Gulf of Elat were compared to determine their relationship to the specific seasonal and geographic distribution patterns of these bacteria. The effects of temperature on growth rate and yield, relative sensitivity to photooxidation, resistance to high salt concentration (8%), and ability to grow in nutrient-poor conditions appear to control these patterns. The winter appearance of Photobacterium fischeri and the succession of winter and summer types of Beneckea harveyi in the eastern Mediterranean are explained by different temperature requirements for growth. Sensitivity to photooxidation explains the disappearance of P. leiognathi, present in the main body of the Gulf of Elat throughout the year, from the shallow coastal strip. B. harveyi is present in this coastal strip which is higher in nutrients and in productivity than the open waters. Competition experiments between B. harveyi and P. leiognathi in batch and continuous culture indicate that the oligotrophic P. leiognathi is outcompeted by B. harveyi in rich and even in relatively poor media. The distribution pattern found in the Bardawil hypersaline lagoon is explained by selection of salinity-resistant mutants of B. harveyi from the Mediterranean Sea.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972 Sep;111(3):682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard M., Lees N. D., Burrows L. S., Kleinhans F. W. Differences in crystal violet uptake and cation-induced death among yeast sterol mutants. J Bacteriol. 1978 Sep;135(3):1146–1148. doi: 10.1128/jb.135.3.1146-1148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eloff J. N., Steinitz Y., Shilo M. Photooxidation of cyanobacteria in natural conditions. Appl Environ Microbiol. 1976 Jan;31(1):119–126. doi: 10.1128/aem.31.1.119-126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica; a model of symbiosis based on bacterial studies. Biol Bull. 1976 Dec;151(3):574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- Yetinson T., Shilo M. Seasonal and geographic distribution of luminous bacteria in the eastern mediterranean sea and the gulf of elat. Appl Environ Microbiol. 1979 Jun;37(6):1230–1238. doi: 10.1128/aem.37.6.1230-1238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


