Abstract
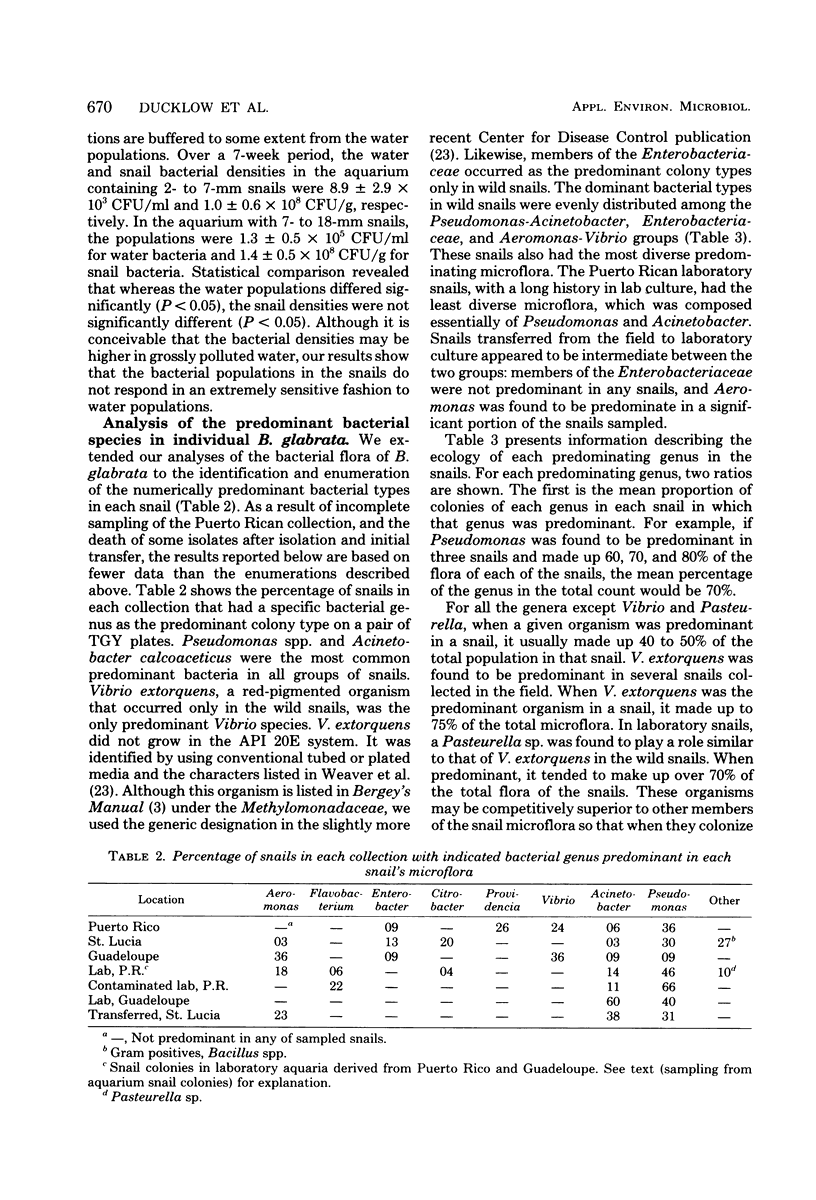
The aerobic heterotrophic bacterial flora in over 200 individuals from 10 wild populations and 3 laboratory colonies of the schistosome vector snail Biomphalaria glabrata was examined. Internal bacterial densities were inversely proportional to snail size and were higher in stressed and laboratory-reared snails. The numerically predominant bacterial genera in individual snails included Pseudomonas, Acinetobacter, Aeromonas, Vibrio, and several members of the Enterobacteriaceae. Enterobacteriaceae seldom predominated in laboratory colonies. Our data suggest that Vibrio extorquens and a Pasteurella sp. tend to predominate in high-bacterial-density snails. These snails may be compromised and may harbor opportunistic snail pathogens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett K. H., Trust T. J. Isolation of salmonellae and other potential pathogens from the freshwater aquarium snail Ampullaria. Appl Environ Microbiol. 1976 May;31(5):635–639. doi: 10.1128/aem.31.5.635-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. J., Etges F. J., Jennelle L. J. The identity and source of a molluscicide-degrading bacterium. Am J Trop Med Hyg. 1966 Jul;15(4):539–543. doi: 10.4269/ajtmh.1966.15.539. [DOI] [PubMed] [Google Scholar]
- CHERNIN E. A method of securing bacteriologically sterile snails (Australorbis glabratus). Proc Soc Exp Biol Med. 1957 Oct;96(1):204–210. doi: 10.3181/00379727-96-23433. [DOI] [PubMed] [Google Scholar]
- CRUZ FILHO O., DIAS E. Bacillus pinottii sp. n. Trans R Soc Trop Med Hyg. 1953 Nov;47(6):581–582. doi: 10.1016/s0035-9203(53)80012-x. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Richards C. S., Popkin T. J. Novel bacterium infecting an African snail. J Bacteriol. 1977 Dec;132(3):950–966. doi: 10.1128/jb.132.3.950-966.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean W. W., Mead A. R., Northey S. T. Aeromonas liquefaciens in the giant African snail, Achantina fulica. J Invertebr Pathol. 1970 Nov;16(3):346–351. doi: 10.1016/0022-2011(70)90150-3. [DOI] [PubMed] [Google Scholar]
- Jobin W. R., Brown R. A., Vélez S. P., Ferguson F. F. Biological control of Biomphalaria glabrata in major reservoirs of Puerto Rico. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):1018–1024. doi: 10.4269/ajtmh.1977.26.1018. [DOI] [PubMed] [Google Scholar]
- Jobin W. R. Control of Biomphalaria glabrata in a small reservoir by fluctuation of the water level. Am J Trop Med Hyg. 1970 Nov;19(6):1049–1054. doi: 10.4269/ajtmh.1970.19.1049. [DOI] [PubMed] [Google Scholar]
- MICHELSON E. H. An acid-fast pathogen of fresh-water snails. Am J Trop Med Hyg. 1961 May;10:423–433. doi: 10.4269/ajtmh.1961.10.423. [DOI] [PubMed] [Google Scholar]
- Richards C. S. Spirochaetes in planorbid molluscs. Trans Am Microsc Soc. 1978 Apr;97(2):191–198. [PubMed] [Google Scholar]
- von Graevenitz A. The role of opportunistic bacteria in human disease. Annu Rev Microbiol. 1977;31:447–471. doi: 10.1146/annurev.mi.31.100177.002311. [DOI] [PubMed] [Google Scholar]


