Abstract
Conventional antibiotics target the growth and the basal life processes of bacteria leading to growth arrest and cell death. The selective force that is inherently linked to this mode of action eventually selects out antibiotic-resistant variants. The most obvious alternative to antibiotic-mediated killing or growth inhibition would be to attenuate the bacteria with respect to pathogenicity. The realization that Pseudomonas aeruginosa, and a number of other pathogens, controls much of their virulence arsenal by means of extracellular signal molecules in a process denoted quorum sensing (QS) gave rise to a new ‘drug target rush’. Recently, QS has been shown to be involved in the development of tolerance to various antimicrobial treatments and immune modulation. The regulation of virulence via QS confers a strategic advantage over host defences. Consequently, a drug capable of blocking QS is likely to increase the susceptibility of the infecting organism to host defences and its clearance from the host. The use of QS signal blockers to attenuate bacterial pathogenicity, rather than bacterial growth, is therefore highly attractive, particularly with respect to the emergence of multi-antibiotic resistant bacteria.
Keywords: quorum sensing, Pseudomonas aeruginosa, quorum-sensing inhibition, cystic fibrosis, biofilms
1. The paradigms of quorum-sensing regulation and infectious diseases
The majority of the published papers on quorum sensing (QS) express the view that the primary function of QS is to enable bacteria to make collective decisions with respect to the expression of a specific set of genes. The cells are not physically aware of the presence and the number of other bacteria (Manefield & Turner 2002), but sense the concentration of the signal molecules, which in turn depends on the cell population density. The principles behind QS signal-mediated gene expression in both Gram-positive and Gram-negative bacteria are shared (Williams et al. 2007), but the molecular mechanisms and signal molecules differ. Gram-negative signalling systems, based on N-acyl- homoserine lactone (AHL) signal molecules, are the most intensively studied examples of QS, and so far more than 70 Gram-negative bacterial species have been reported to produce AHLs (Fuqua et al. 2001; Taga & Bassler 2003). AHL production is considered indicative of the presence of functional QS regulatory circuits.
Most diseases in which QS regulation plays an important role originate from infections caused by opportunistic pathogens. These infections often become chronic as a consequence of the bacterium adopting the sessile, biofilm mode of growth. This is in contrast to acute infections in which the bacteria proliferate in a planktonic state. The dense biofilm, including the surrounding extracellular polysaccharide (EPS) matrix, provides ideal conditions for the accumulation of QS signal molecules, which triggers the onset of QS-regulated gene expression which in turn affects the responses of the host immune system (Nilsson et al. 2001). In addition, the vasorelaxant properties of the Pseudomonas aeruginosa AHL, N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) also suggest that the blood supply to the infection site might be affected by the biofilm bacteria (Lawrence et al. 1999). The sessile, biofilm mode of growth is recognized to be important in all implant-related infections, and with every foreign medical device inserted into a patient there is a potential risk that a bacterial biofilm will form over time on the artificial surface.
2. Pseudomonas aeruginosa
The most intensively studied bacterium employing AHL-based QS is undoubtedly P. aeruginosa. This is the most common bacterium found in nosocomial and life-threatening infections of immune compromised patients (van Delden & Iglewski 1998). It causes a variety of acute and chronic infections listed by van Delden & Iglewski (1998): nosocomial pneumonia cases, hospital-acquired urinary tract infections, surgical wound infections, bloodstream infections, infection of neutropenic patients, ventilator-associated pneumonia in intubated patients, burn wound infections, etc. It is particularly known for its involvement in chronic infections of the respiratory pathways including diffuse panbronchiolitis, bronchiectasia and cystic fibrosis (CF; Hoiby 1974; Koch & Hoiby 1993; Frederiksen et al. 1997; Kobayashi 2005). Pseudomonas aeruginosa is a Gram-negative bacillus, belonging to the genus Pseudomonas (γ-proteobacteria). A characteristic of the genus Pseudomonas is simple growth requirements and tolerance to a wide range of temperatures (4–42°C), and it is one of the most diverse and ecologically significant groups of bacteria that are known. The complete genome of P. aeruginosa PA01 consists of 5770 open reading frames (ORFs; Stover et al. 2000). This makes it one of the largest known bacterial genomes, around 50% larger than Escherichia coli K-12. With respect to genetic complexity, P. aeruginosa resembles simple eukaryotes such as Saccharomyces cerevisiae.
Pseudomonas aeruginosa can be isolated from most environments—soils, marshes, coastal marine habitats, plants and mammal tissues (Stover et al. 2000). In hospital environments, it grows in moist reservoirs such as food, cut flowers, sinks, toilets, floor mops, dialysis equipment and even in disinfectant solutions (Murray et al. 2002). Pseudomonas aeruginosa emerged as a human pathogen in the past century, probably by the development of available niches as a result of successful eradication of other pathogens by antibiotics and disinfectants.
Pseudomonas aeruginosa produces a wide range of virulence factors, which makes it highly pathogenic for susceptible patients. The arsenal of virulence factors produced by P. aeruginosa includes both cell-associated and extracellular products. Among these are lipopolysaccharide (Tang et al. 1996); the two ADP ribosylating enzymes, exotoxin A (Vasil et al. 1989) and exoenzyme S (Nicas et al. 1985); the exoproteases elastase (Woods et al. 1982), LasA (Preston et al. 1997) and alkaline protease (Howe & Iglewski 1984); a haemolytic and a non-haemolytic phospholipase C (Vasil et al. 1991); the siderophores pyochelin and pyoverdine (Cox 1982); the surface-associated polysaccharide, alginate (Pedersen et al. 1992); and pilus-associated adhesins responsible for binding to specific epithelial cell receptors (Tang et al. 1995). To control gene expression, P. aeruginosa employs 468 transcriptional regulators which correspond to 8.4% of the predicted total number of ORFs (Stover et al. 2000). The pathogen also uses more than 60 two-component systems which are essential for gene regulation. The production of many of the key virulence factors is controlled by QS (van Delden & Iglewski 1998; Rumbaugh et al. 2000; Williams et al. 2000).
3. Quorum sensing of P. aeruginosa
The two AHL-based QS systems of P. aeruginosa comprise LuxR and LuxI homologues with specific signal preferences, 3-oxo-C12-HSL for the las system and N-butanoylhomoserine lactone (C4-HSL) for the rhl system (Pesci et al. 1997; figure 1). The complexity and plasticity of QS in P. aeruginosa is illustrated by the fact that three individual research groups identified a number of differences in the composition of the QS regulon (Hentzer et al. 2003; Schuster et al. 2003; Wagner et al. 2003). The most attractive explanation is that much of this is probably attributed to the differences in media, growth conditions and data evaluation; but we cannot rule out the possibility that this is also a matter of local strain differences. Hentzer et al. (2003) compared the results of all the three groups and identified 77 QS-regulated genes which were common for all three studies. These 77 genes have been denoted the ‘the general QS regulon’ and are probably non-conditionally expressed.
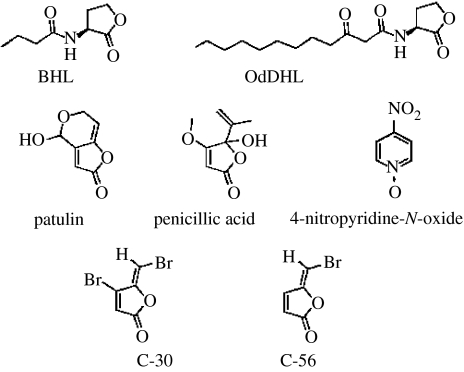
Figure 1.
The two main AHL signal molecules of P. aeruginosa, 3-oxo-C12-HSL and C4-HSL, the natural fungal QSI compounds, patulin and penicillic acid, and the synthetic halogenated furanones C-30 and C-56 and 4-nitropyridine-N-oxide.
4. The biofilm mode of growth
The success of P. aeruginosa is attributed to a number of survival strategies among which are its ability to form biofilms and adapt to almost any environmental niche. Often, the only cure for a chronic infection is the surgical removal of the infected implant or organ. A biofilm definition which applies to both in vitro and in vivo conditions was offered by Donlan & Costerton (2002). According to this, a biofilm is a sessile community characterized by cells that are irreversibly attached to a surface or interface, or to each other, which are imbedded in a matrix of EPS and exhibit an altered phenotype with respect to growth rate and gene transcription, as compared with planktonic bacteria. This definition also extends to pulmonary infections in CF patients, as well as patients with chronic wounds where the biofilms are associated with moist tissue rather than hard, artificial surfaces.
5. The relation between quorum sensing and biofilm development in P. aeruginosa
The involvement of QS in organized surface-associated behaviour of bacteria was first described for S. liquefaciens by Eberl et al. (1996). Later, QS was implicated in the process of biofilm formation by P. aeruginosa (Davies et al. 1998b; Glessner et al. 1999). Davies et al. (1998b) reported that the las system was indispensable for normal biofilm development, including differentiation that would lead to the formation of the characteristic mushroom and tower structures observed in in vitro biofilms. Apparently, this is a subtle phenotype as indicated by observations made by Heydorn et al. (2002), Hentzer et al. (2003), Hentzer et al. (2004) and Bjarnsholt et al. (2005a,b). They showed that QS-deficient strains initially form densely packed biofilms similar to the wild-type (WT). The QS mutant biofilm even produced mushroom structures similar to the WT with glucose as the carbon source. Hentzer et al. (2004) have shown that, in comparison to WT, aged (10 days or more) QS-deficient biofilms differed in size and structural stability. Likewise, Bjarnsholt et al. (2005a) showed that a QS-deficient biofilm appears more flexible than the WT counterpart. This is possibly due to the QS-regulated production of extracellular DNA (Allesen-Holm et al. 2005), which possibly acts as scaffolding and stabilizes the three-dimensional structure of the biofilm. Hence, apparently the involvement of QS signalling in the biofilm-forming process is a conditional effect rather than involvement in a biofilm-specific developmental programme (Kjelleberg & Molin 2002). We believe that much of the initial confusion was caused by the use of QS mutants that had accumulated secondary mutations contributing to misinterpretations of the QS effect (Beatson et al. 2002).
Recent analysis based on transcriptomics revealed that, even at the early stages, the bulk of biofilm cells (in once-through flow chambers) express genes in a pattern that is reminiscent of gene expression seen in early stationary phase of planktonic cells (Hentzer et al. 2005). Accordingly, the majority of QS regulated genes are expressed within 16 h of inoculation in minimal medium; the authors even identified a number of QS-regulated genes that were preferentially expressed in the biofilm mode of growth, including genes involved in iron acquisition and metabolism. However, other research groups have failed to identify a similar QS-specific biofilm regulon and, taken together, there is at present no evidence that a biofilm-specific programme is operating in P. aeruginosa during conditions of sessile growth and biofilm formation. The involvement of QS in the development of increased biofilm tolerance to some antimicrobial treatments is therefore something of a paradox. Davies et al. (1998a) demonstrated that a QS-deficient lasI mutant of P. aeruginosa formed biofilms that were more susceptible to biocides. Likewise, biofilms formed by a lasR, rhlR double mutant of P. aeruginosa were much more prone to killing by tobramycin and hydrogen peroxide (Bjarnsholt et al. 2005a), as well as by ciprofloxacin and ceftizidime (T. Bjarnsholt & M. Givskov 2006, unpublished observations), than were biofilms formed by the WT. The QS-regulated genes that are involved in this increased tolerance remain to be identified; however, some multidrug efflux pumps are known to be QS regulated.
Our view is that biofilm development by P. aeruginosa is mainly governed by adaptive responses, and this view is strengthened by the finding that biofilm development is associated with a large number of genes required for anaerobic respiration (Hentzer et al. 2005). It should be emphasized that this does not necessarily apply to other biofilm-forming bacteria. Hence, QS is an actively regulating gene expression in the P. aeruginosa biofilms, as it is in the planktonic state; however, it is not required for biofilm formation per se but rather as a consequence of increased cell population density and growth physiology; but there are differences. Interestingly, Hentzer et al. (2005) found an induced QS-regulated expression of PvdQ in biofilms, compared with planktonic growth. PvdQ is one of at least two acylase enzymes that degrade 3-oxo-C12-HSL (Huang et al. 2003, 2006). This result may provide an explanation for the previous finding that the level of long acyl AHLs is greatly reduced in P. aeruginosa biofilm cells, as compared with their planktonic counterparts. Another ongoing discussion is the relevance of biofilm structures for antibiotic tolerance, because both the WT and QS-deficient in vitro biofilms of P. aeruginosa make differentiated biofilm structures, but exhibit different tolerances to antibiotics and phagocytosis (see § 7). We believe there is no direct correlation between the three-dimensional structure and tolerance to various antimicrobial treatments. A QS mutant may form the same three-dimensional structure as the WT, dependent on carbon source, but is less tolerant to a range of antimicrobial agents. Haagensen et al. (2007) have shown that the ‘mushrooms’ consist of different bacterial subpopulations, in terms of growth and motility, and these subpopulations differ in antimicrobial tolerance. We believe that this is rather a matter of different physiology and not structure per se since biofilms grown on citrate as the carbon source show the same subpopulations and antimicrobial properties, but lack the differentiated three-dimensional structure.
6. Cystic fibrosis, a prominent biofilm disease
Cystic fibrosis(CF) is a monogenic autosomal recessive multi-organ disease, the most common lethal inherited disease in Caucasians (Koch 2002). Worldwide, the incidence of the gene defects varies from 1 : 32 000 to 1 : 2000 live births in Caucasians (Gibson et al. 2003). The genetic cause of CF was identified in 1989 as a defect in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, located on chromosome 7 (Kerem et al. 1989; Riordan et al. 1989; Rommens et al. 1989). The defect in the CFTR causes a decrease in epithelial chloride secretion and an increase in sodium absorption. In the lung, this results in viscous dehydrated mucus which is very difficult to clear mechanically, e.g. by coughing. The background which renders the mucus abnormally viscous is the chronic depletion of the periciliary liquid layer (Boucher 2002; Donaldson & Boucher 2003). Since the non-inflammatory defence mechanism (mucociliary clearance) is impaired, recurring, and later chronic, bacterial lung infection occurs (Knowles & Boucher 2002; Gibson et al. 2003).
From their early childhood, CF patients suffer from acute infections of many different bacteria, the most common being Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, P. aeruginosa, the Burkholderia cepacia complex and Stenotrophomonas maltophilia (Bauernfeind et al. 1987; Saiman 2004a). Haemophilus influenzae and S. aureus predominate early in life, but later S. aureus and P. aeruginosa become the dominating infectious organisms in the CF lung. Up to 80% of young adults suffering from CF are chronically infected with P. aeruginosa (Bauernfeind et al. 1987; Gilligan 1991; Koch & Hoiby 2000; Collier et al. 2002; Koch 2002).
Pseudomonas aeruginosa recurrently colonizes CF patients and in this early phase, no symptoms of infection are observed in the lower respiratory tract. It is not until later that the patients enter a phase of chronic bronchopulmonary inflammation, antibody development and deteriorating pulmonary function. It is now evident that it is not the infecting bacteria per se, but rather a prolonged immune complex-mediated inflammatory response dominated by polymorphonuclear leukocytes (PMNs), which causes the tissue damage, necrosis of the lung tissue and eventually the death of the patient as a result of the loss of pulmonary function (Koch & Hoiby 1993; Gibson et al. 2003).
The first evidence of biofilm formation in the CF lung was presented by Hoiby who discovered ‘heaps’ (microcolonies) of P. aeruginosa in the sputum of CF patients (Hoiby 1977). By means of electron microscopy, in the post-mortem of P. aeruginosa infected CF lungs, Lam et al. (1980) showed bacterial microcolonies in alveoli. Based on immunohistopathology from five CF lungs, Baltimore et al. (1989) identified P. aeruginosa microcolonies in post-mortem of CF lungs, stating the location of the bacteria was endobronchial, mainly in the small (less than 1 mm) airways. Another immunohistopathology quantitative study revealed that the bacterial density in CF airways is highest in the bronchi (Potts et al. 1995). The P. aeruginosa biofilm is not always surface associated (Worlitzsch et al. 2002; Hall-Stoodley et al. 2004; T. Bjarnsholt & M. Givskov 2006, unpublished observations) but pertains to microcolonies in the CF mucus surrounded by numerous leukocytes. Whether a non-surface-attached microcolony is considered as a biofilm or not, it shares some of the same characteristics with a sessile film. We believe that the matrix structure and physiology are obvious traits shared between surface and non-surface-attached biofilms.
7. Quorum sensing and chronic P. aeruginosa pulmonary infections
Tang et al. (1996) discovered that QS and several QS-controlled virulence factors were necessary for establishing a pulmonary infection in neonatal mice. A possible link between QS and the chronic P. aeruginosa lung infection in CF patients became apparent when Storey et al. (1998) discovered a correlation between lasR transcription and the transcription of lasA, lasB and toxA in CF sputa. In 2000, it was reported that a number of P. aeruginosa isolates from CF patients were capable of producing QS signal molecules when grown in vitro (Geisenberger et al. 2000), and Wu et al. (2000) demonstrated that cell-to-cell signalling takes place in lung tissue of P. aeruginosa infected mice. Wu et al. (2001) showed in a murine model of chronic lung infection and Pearson et al. (2000) in an acute neonatal mouse model that a lasI rhlI mutant of P. aeruginosa is less virulent and is cleared faster than the WT. These findings were confirmed with an infectious rat model (Wu et al. 2004a). Rumbaugh et al. (1999) used a burned mouse model of infection to show that QS-deficient mutants of P. aeruginosa were less virulent than the WT in vivo.
There then followed the detection of QS signals in sputum (Erickson et al. 2002; Middleton et al. 2002) and directly in the lung tissue of CF patients (Favre-Bonte et al. 2002). The ratio of the signal molecules was found to vary from the CF lung to the CF sputum. Erickson et al. (2002) detected both 3-oxo-C12-HSL and C4-HSL in the sputum samples, but the C4-HSL signal molecule was less frequent when compared with the 3-oxo-C12-HSL signal molecule. On the other hand, Favre-Bonte et al. (2002) reported that they detected 100 times more C4-HSL than 3-oxo-C12-HSL in all the CF-lung samples. This correlates with the ex vivo biofilm results reported by Singh et al. (2000) and the above mentioned observation of increased QS-controlled expression of PvdQ in in vitro biofilms which specifically eliminate 3-oxo-C12-HSL (Hentzer et al. 2005).
The number of bacteria can exceed 108 colony forming units (CFU) per ml in CF sputum (Bauernfeind et al. 1987), i.e. the density of bacteria in sputum should be sufficient to cause induction of QS-controlled gene expression (Hentzer et al. 2003). However, in the respiratory zone of the CF lung, the discrete microcolonies (Worlitzsch et al. 2004) may not contain the in vitro established quorum size. Limitations of space will contribute to the increased local cell concentration; taken together with the observation by van Delden et al. (2001), who reported induction of QS-regulated genes independent of cell density, but in response to the lack of nutrient availability, this might contribute to QS-regulated gene expression. Whether or not the QS-regulated genes are expressed in these small microcolony biofilms needs further investigation.
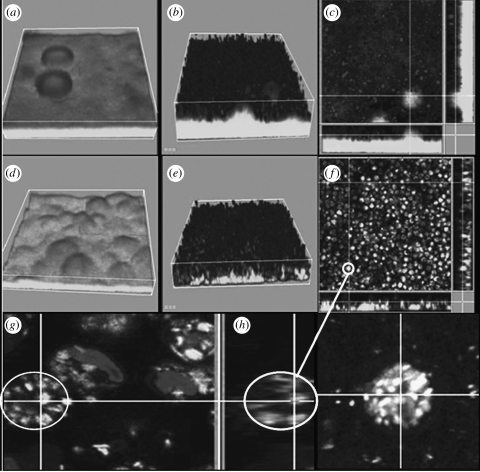
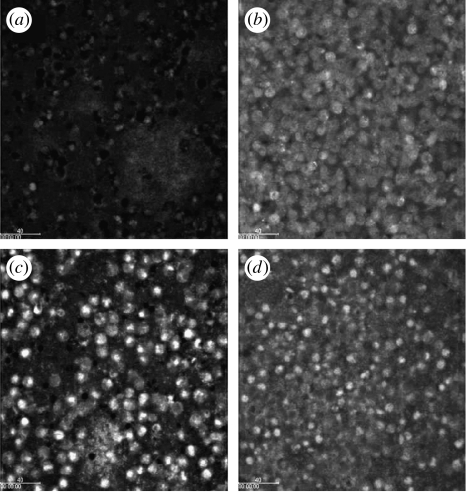
The impact of QS on the severity of infection was recently confirmed by Bjarnsholt et al. (2005a) using a lasR rhlR mutant in a pulmonary mouse model. Importantly, although the QS deficiency provoked an initially higher degree of inflammation, the mice had an overall better health status, significantly reduced mortality and the lasR rhlR mutant bacteria were cleared significantly faster than their WT counterparts. Histopathological data showed a higher influx of PMNs in the lungs of the mice infected with the QS-deficient strain. However, the concentration of the PMN chemoattractant MIP-2 (the mouse analogue of IL-8) was higher for the WT infected mice. In order to explain this apparent paradox, we hypothesized that P. aeruginosa, by means of a QS-controlled mechanism, is capable of attenuating the PMN defence. This hypothesis has gained momentum from in vitro investigations. We observed that PMNs inoculated on the top of a WT P. aeruginosa biofilm were unable to graze on the bacteria. In contrast, PMNs inoculated on top of a QS-deficient biofilm readily grazed the biofilm bacteria (figure 2). Since the PMNs in contact with the WT biofilm appeared to be ‘paralysed’, we investigated whether the PMNs were responding to the bacteria at all. As part of an antibacterial cocktail, the PMNs will form oxygen radicals, some of which will give rise to production of H2O2. This is known as the oxidative burst and was also blocked by a QS-controlled mechanism since, in contrast to a QS-deficient biofilm, no H2O2 production was detected on exposure to a WT biofilm (figure 3).
Figure 2.
QS-dependent tolerance of P. aeruginosa biofilms towards PMNs. Three-day-old biofilms of wild-type P. aeruginosa (a–c) and a ΔlasR rhlR mutant (d–g), both expressing GFP as a tag (grey), were exposed to PMNs for about 2 and 5 h. The PMNs appear black due to SYTO-62 staining. (b) Three-dimensional projection and (c) cross-section showing the wild-type biofilm with PMNs on top. (e) Three-dimensional projection and (f) cross-section showing the ΔlasR rhlR mutant fully penetrated by PMNs and the disappearance of much of the biomass. As seen from the enlargement of PMNs exposed to a QS-deficient biofilm frame (g) and isolated PMNs (h), the green fluorescent bacteria (grey) are attached to and phagocytosed by the PMNs. (Reproduced with permission from Bjarnsholt et al. (2005a).)
Figure 3.
PMN activation measured by oxidative burst. Three-day old biofilms of wild-type P. aeruginosa and the ΔlasR rhlR mutant were exposed to PMNs for 2 h. The oxidative burst was visualized by the green fluorescence (light grey) emitted when 123-DHR is oxidized to 123-rhodamine due to production of H2O2. (a) Wild-type; (c) ΔlasR rhlR mutant. (b) Furanone C-30 and (d) Garlic extract mediated activation of PMNs present on a wild-type biofilm. The biofilm was grown for 3 days in the presence of 10 mM C-30 or 2% garlic extract. The PMNs fluoresce green (indicative of oxidative burst) compared with the PMNs in (a). (Reproduced with permission from Bjarnsholt et al. (2005a).)
8. Screens for quorum-sensing blockers
As described above, QS appears to play an important role in the ability of P. aeruginosa to cause disease, but direct involvement in pulmonary infection in CF is still largely based on circumstantial evidence. However, in our view, the present knowledge justifies the pursuit of QS blockage as a possible treatment of a variety of P. aeruginosa-based infections. In addition, an increasing number of clinical isolates show multiple resistances to conventional antibiotics (Norrby et al. 2005) which emphasizes the urgency of speeding up research and development in this particular field. The typical screen for QS inhibitor (QSI) activity involves a gene fusion of a QS-regulated promoter to a reporter gene. In the presence of exogenously added AHL signal molecules, these reporters are activated to express their corresponding reporters. If, in addition to AHL, exogenous QSI activity is also present, expression of the reporters is reduced or abolished. This screen is considered positive if the reporter gene is not expressed. Unfortunately, a compound or extract that slows growth, such as sublethal concentrations of antibiotics, will affect protein synthesis and, in turn, reduce the signal from the reporter; this can lead to the scoring of a ‘false positive’.
To circumvent these problems, Rasmussen et al. (2005a) developed an alternative system in which the growth of the screening bacterium indicates the presence of QSI activity. Briefly, a QS-regulated promoter is fused to a ‘killer’ gene, the product of which is detrimental to the host cells. Established in a background unable to direct the synthesis of signal molecules, the presence of exogenously added AHL enables QS and promotes the expression of the killer gene which in turn kills the host cells. However, the presence of QSI molecules blocks stimulation of the QS-regulated ‘killer’ gene which, as a consequence, rescues the host cells and enables growth. This diminishes the number of false positives identified in a high through-put setup. Genetically modified bacteria, termed QSI selectors, based on both the lux and the las/rhl QS systems have been developed (Rasmussen et al. 2005a) and performed as an agar passive diffusion assay. This enables rapid verification of the inhibitory, as well as toxic, properties of a particular test compound or extract. However, a possible QSI candidate needs to be tested further, to determine if it causes inactivation of the AHL or of AHL synthesis, or blocks the signal molecule-binding site of the LuxR homologue proteins.
9. Blockers of quorum sensing
The first examples of QS-blocking compounds came from the marine environment. It was shown by de Nys et al. (1993) that the Australian macroalga, Delisea pulchra, produces a range of halogenated furanones. A characteristic of halogenated furanones is their antifouling property, which is obvious from field experiments. Givskov et al. (1996) and Kjelleberg et al. (1997) offered the first demonstration that these secondary metabolites specifically inhibit bacterial QS. The exact mechanism of inhibition is unknown, but Western-blot analysis indicates that the furanones are able to promote rapid turnover of the LuxR protein, reducing the amount of protein available to interact with AHL and act as transcriptional regulator (Manefield et al. 2002).
Since the natural furanones do not efficiently block QS in P. aeruginosa, a series of synthetic analogues were made. A structure–function analysis of several furanones with varying side chain length and substitutions on the furanone ring indicated that compounds without side chains, but with electronegative substituents on the furanone ring, were effective in inhibiting the QS systems of P. aeruginosa (Anthony et al. 1997; Hentzer et al. 2002; Manefield et al. 2002; Hjelmgaard et al. 2003). Among these, compounds C-30 (Hentzer et al. 2003) and C-56 (Hentzer et al. 2002) showed QSI potential in P. aeruginosa (figure 1). They used a lasB-based AHL monitor to detect the QS regulation of in vitro biofilms. Control over lasB expression is transcriptionally governed by LasR and RhlR (Pearson et al. 1997). Planktonic and biofilm grown P. aeruginosa showed reduced green fluorescence in the presence of furanone C-56 and revealed efficient penetration through the entire biofilm structure. The concentrations of furanone C-56 used were neither growth inhibitory nor interfered with the basal metabolism. Hentzer et al. (2003) showed that biofilms grown with and without furanone C-30 were not significantly different in biofilm formation and biomass. However, the furanone C-30 treated biofilm was significantly less tolerant to treatment with tobramycin (100 μg ml−1). It has subsequently been shown by means of transcriptomic analysis that C-30 specifically inhibits QS-controlled genes in P. aeruginosa (Hentzer et al. 2003). Among 93 genes, 1.7% of the P. aeruginosa genome are significantly affected by the presence of furanone C-30 of which 80% are QS controlled.
The QSI effect of both furanones C-30 and C-56 has been demonstrated in a pulmonary mouse model (Hentzer et al. 2003; Wu et al. 2004b). The bacteria were cleared significantly faster in mice treated with 1 μg furanone per gram body mass. The potential of furanone C-30 for treatment of infectious diseases has also been demonstrated in a fish-farming model system. In a cohabitant experiment of Vibrio anguillarum infection, significantly reduced mortality of rainbow trout was observed on treatment with as little as 25 ng ml−1 furanone C-30 (Rasch et al. 2004), indicating QS blockage as a useful alternative to antibiotics in fish farming.
Macrolides, such as azithromyzin, have been reported to inhibit QS (Tateda et al. 2001). Azithromyzin reduces the production of several of the virulence factors of P. aeruginosa, such as elastase and rhamnolipids. A reduction of lasI and rhlI expression was also reported; however, in our hands, we are unable to reproduce effects on QS-controlled transcription without using concentrations that also affect growth rate. It is our view that the effect on QS-controlled virulence, such as alginate synthesis (Ichimiya et al. 1996), is caused by pleiotropic effects. The important clinical aspect, however, is that administration of this drug improves the respiratory function in CF patients (Saiman 2004b; Southern & Barker 2004).
Recently, a number of papers describing additional natural QSI compounds have been published (Bjarnsholt et al. 2005b; Rasmussen et al. 2005a,b; figure 1). They have been found in food, herbal and fungal sources, and tested on in vitro biofilms and in a pulmonary mouse model of infection. The presence of QSI compounds in natural foods is extremely interesting because, in most cases, vegetables and herbs are non-toxic to humans and are readily available. Of particular interest is garlic, since it is a widely used spice. Garlic is renowned for its variety of antifungal, anticancer and antimicrobial activities. However, the previous acknowledged antimicrobial activities have been related to the presence of growth inhibitory compounds such as allicin and its derivatives (Ankri & Mirelman 1999). Synthesis of chemically pure allicin has shown that this is not the active QSI ingredient (T. Bjarnsholt & M. Givskov 2006, unpublished results).
In a recent study by Bjarnsholt et al. (2005b), P. aeruginosa was grown in vitro in continuous culture, once-through, flow chambers with and without garlic extract. The garlic-treated biofilm was susceptible to both tobramycin treatment and grazing of PMNs. The PMNs showed an increased activation when incubated on the garlic treated biofilm (figure 3). The results of this in vitro investigation indicate that a QS inhibitory extract of garlic renders P. aeruginosa biofilms sensitive to tobramycin, phagocytosis and the PMN respiratory burst. For an in vivo infection treatment study, the pulmonary mouse model was used. Mice were treated with garlic extract or placebo for 7 days, with the initial 2 days being prophylactic before P. aeruginosa was instilled in the left lung of the mice. The garlic treatment of the infected mice resulted in a significantly improved clearing of the infecting bacteria. Concurrently with the results obtained with the QS mutants, we initially observed an increased endobronchial influx of PMNs and a higher degree of inflammation in the mice treated with the garlic extract. Consequently, blockage of QS by the garlic treatment led to significantly faster clearing of the bacteria and a strongly reduced mortality. These data support the view that the inflammation detected in the lungs of the garlic-treated mice are the adequate response to the intruders. The QS-controlled paralysing mechanism reduces, compared with ‘normal’, the inflammation in the placebo group of mice. This is based on the observation that the concentration of the PMN chemoattractant MIP-2 is lower in the garlic-treated group when compared with the placebo group. In addition, there was no difference in the concentration of myeloperoxidase, a quantitative measurement of PMNs, in the two groups. The same observations were made on using QS mutants in the in vivo model. These observations indicate that a sufficient number of viable and active PMNs are present in P. aeruginosa infected lungs only when QS is blocked.
10. The future and perspectives
As implied in the previous sections, the biofilm mode of growth and QS-controlled gene expression play important roles in chronic bacterial infections. It has recently been stated by the National Institutes of Health in the USA that ‘More than 70 per cent of the bacteria that cause hospital-acquired infections are resistant to at least one of the drugs most commonly used to treat them’ (National Institutes of Health 2004). Consequently, we need a new approach to control or interfere with this fundamental microbial activity. Since the virulence of many bacteria is regulated by QS, it is an obvious drug target. This covers the production of virulence factors, antibiotic resistance and modulation of the host immune system. The proofs of concept have already been offered by Hentzer et al. (2003), Rasch et al. (2004), Wu et al. (2004b) and Bjarnsholt et al. (2005b). A caveat to this is that the halogenated furanones exhibit both toxic side effects and possible carcinogenic properties, which make them particularly unsuitable for human pharmaceutical usage. This highlights the necessity of focusing on further drug isolation and development of pharmaceutically relevant QSI drug candidates.
The QS system of P. aeruginosa is a potential drug target if the findings presented in this review can be extrapolated to the human lung and other sites of chronic infection. Most of the literature is based on the use of non-mucoid P. aeruginosa which predominate during the early stages of the pulmonary infection seen in CF patients. Several considerations will have to be made using QSI drugs in the treatment of CF patients and other patient groups. Firstly, the point of no return in the CF lung might be as early as when the infecting P. aeruginosa start to interfere with the antimicrobial properties of PMNs. It has been stated that the PMNs become frustrated in their attempt to graze on the biofilm. However, based on the results presented in our previous publications, we suggest that the PMNs are impaired by a QS-regulated phenotype of P. aeruginosa. It is possible that the PMNs become necrotic and lyse before the macrophages scavenge them and/or the macrophages are impaired as well. The PMNs are beneficial if they work properly, removing the intruders, but obviously very harmful as seen in the CF lung, if they become functionally impaired (Konstan et al. 2003). The magnitude of the oxidative burst varies between CF patients (Fruhwirth et al. 1998). This correlates with the finding that strains of P. aeruginosa isolated from the sputum of some chronically infected CF patients (Geisenberger et al. 2000) produce lower amounts of signal molecules.
The obvious synergistic activities of QSI drugs make it even more relevant to consider QS blockage as part of an early interventionist chemotherapy. Upon prophylactic administration of QSI, the CF patients would be expected to experience only recurrent acute infections which are sensitive to the host defence system and conventional antibiotics, and therefore do not reach the point of no return leading to the establishment of chronic infection. Chronically infected CF patients carrying a reservoir of mucoid P. aeruginosa may not be cured by QSI treatment, but the deterioration in lung function might be halted. New focal microcolonies of mucoid P. aeruginosa, causing the acute damaging inflammation of the respiratory zone in the CF lung, might not ‘paralyse’ the PMNs, since QS can be blocked by the chemotherapy. The administration of QSIs, antibiotics and adequate numbers of PMNs might very well reduce the collateral damage caused by the PMN activity and also promote eradication of the infecting bacteria. Although CF patients still have the impaired CFTR gene, the activity of this transmembrane conductor might partly be restored by treatment with other small molecule drugs (Rubenstein 2005). Hopefully, in the near future, physicians will be able to treat patients suffering from chronic bacterial infections in diseases such as CF and extend their lifespan closer to normal, using chemotherapies which rely and stimulate the synergistic actions of QSI drugs, conventional antibiotics, putative CFTR restoration and the proper activity of the PMN defences.
Acknowledgments
This work was supported by grants from the Danish Technical Research Council to M.G., the Villum Kann-Rasmussen Foundation to M.G. and the Centre for Elimination of Biofilms on Medical Equipment.
Footnotes
One contribution of 12 to a Theme Issue ‘Bacterial conversations: talking, listening and eavesdropping’.
References
- Allesen-Holm M, Barken Bundvig K, Yang L, Klausen M, Webb J.S, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2005;54:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi: 10.1016/s1286-4579(99)80003-3. doi:10.1016/S1286-4579(99)80003-3 [DOI] [PubMed] [Google Scholar]
- Anthony M, Kjelleberg S, Kumar N, de Nys R, Read R, Stainberg P. Reinvestigation of the sulfuric acid-catalysed cyclisation of brominated 2-alkylllevulinic acids to 3-alkyl-5-methylene-2(5H)-furanones. Tetrahedron. 1997;53:15 813–15 826. doi:10.1016/S0040-4020(97)10034-5 [Google Scholar]
- Baltimore R.S, Christie C.D, Smith G.J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am. Rev. Respir. Dis. 1989;140:1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A, Bertele R.M, Harms K, Horl G, Jungwirth R, Petermuller C, Przyklenk B, Weisslein-Pfister C. Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection. 1987;15:270–277. doi: 10.1007/BF01644137. doi:10.1007/BF01644137 [DOI] [PubMed] [Google Scholar]
- Beatson S.A, Whitchurch C.B, Semmler A.B, Mattick J.S. Quorum sensing is not required for twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 2002;184:3598–3604. doi: 10.1128/JB.184.13.3598-3604.2002. doi:10.1128/JB.184.13.3598-3604.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T, et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005a;151:373–383. doi: 10.1099/mic.0.27463-0. doi:10.1099/mic.0.27463-0 [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology. 2005b;151:3873–3880. doi: 10.1099/mic.0.27955-0. doi:10.1099/mic.0.27955-0 [DOI] [PubMed] [Google Scholar]
- Boucher R.C. An overview of the pathogenesis of cystic fibrosis lung disease. Adv. Drug Deliv. Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. doi:10.1016/S0169-409X(02)00144-8 [DOI] [PubMed] [Google Scholar]
- Collier D.N, Anderson L, McKnight S.L, Noah T.L, Knowles M, Boucher R, Schwab U, Gilligan P, Pesci E.C. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 2002;215:41. doi: 10.1111/j.1574-6968.2002.tb11367.x. doi:10.1111/j.1574-6968.2002.tb11367.x [DOI] [PubMed] [Google Scholar]
- Cox C.D. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect. Immun. 1982;36:17–23. doi: 10.1128/iai.36.1.17-23.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.G, Parsek M.R, Pearson J.P, Iglewski B.H, Costerton J.W, Greenberg E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998a;280:295–298. doi: 10.1126/science.280.5361.295. doi:10.1126/science.280.5361.295 [DOI] [PubMed] [Google Scholar]
- Davies D.G, Parsek M.R, Pearson J.P, Iglewski B.H, Costerton J.W, Greenberg E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998b;280:295–298. doi: 10.1126/science.280.5361.295. doi:10.1126/science.280.5361.295 [DOI] [PubMed] [Google Scholar]
- de Nys R, Wright A.D, Konig G.M, Sticher O. New halogenated furanones from the marine alga Delisea pulchra. Tetrahedron. 1993;49:11 213–11 220. doi:10.1016/S0040-4020(01)81808-1 [Google Scholar]
- Donaldson S.H, Boucher R.C. Update on pathogenesis of cystic fibrosis lung disease. Curr. Opin. Pulm. Med. 2003;9:486–491. doi: 10.1097/00063198-200311000-00007. doi:10.1097/00063198-200311000-00007 [DOI] [PubMed] [Google Scholar]
- Donlan R.M, Costerton J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. doi:10.1128/CMR.15.2.167-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl L, et al. Involvement of N-acyl-l-hormoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol. Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. doi:10.1111/j.1365-2958.1996.tb02495.x [DOI] [PubMed] [Google Scholar]
- Erickson D.L, Endersby R, Kirkham A, Stuber K, Vollman D.D, Rabin H.R, Mitchell I, Storey D.G. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 2002;70:1783–1790. doi: 10.1128/IAI.70.4.1783-1790.2002. doi:10.1128/IAI.70.4.1783-1790.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre-Bonte S, Pache J.C, Robert J, Blanc D, Pechere J.C, van Delden C. Detection of Pseudomonas aeruginosa cell-to-cell signals in lung tissue of cystic fibrosis patients. Microb. Pathog. 2002;32:143–147. doi: 10.1006/mpat.2001.0487. doi:10.1006/mpat.2001.0487 [DOI] [PubMed] [Google Scholar]
- Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr. Pulmonol. 1997;23:330–335. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. doi:10.1002/(SICI)1099-0496(199705)23:5<330::AID-PPUL4>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- Fruhwirth M, Ruedl C, Ellemunter H, Bock G, Wolf H. Flow-cytometric evaluation of oxidative burst in phagocytic cells of children with cystic fibrosis. Int. Arch. Allergy Immunol. 1998;17:270–275. doi: 10.1159/000024022. [DOI] [PubMed] [Google Scholar]
- Fuqua C, Parsek M.R, Greenberg E.P. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. doi:10.1146/annurev.genet.35.102401.090913 [DOI] [PubMed] [Google Scholar]
- Geisenberger O, Givskov M, Riedel K, Hoiby N, Tummler B, Eberl L. Production of N-acyl-l-homoserine lactones by P. aeruginosa isolates from chronic lung infections associated with cystic fibrosis. FEMS Microbiol. Lett. 2000;184:273–278. doi: 10.1111/j.1574-6968.2000.tb09026.x. [DOI] [PubMed] [Google Scholar]
- Gibson R.L, Burns J.L, Ramsey B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. doi:10.1164/rccm.200304-505SO [DOI] [PubMed] [Google Scholar]
- Gilligan P.H. Microbiology of airway disease in patients with cystic fibrosis. Clin. Microbiol. Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P.D, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glessner A, Smith R.S, Iglewski B.H, Robinson J.B. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of twitching motility. J. Bacteriol. 1999;181:1623–1629. doi: 10.1128/jb.181.5.1623-1629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagensen J.A, Klausen M, Tolker-Nielsen T, Ernst R.K, Miller S.I, Molin S. Differentiation and distribution of colistin/SDS tolerant cells in Pseudomonas aeruginosa flow-cell biofilms. J. Bacteriol. 2007;189:28–37. doi: 10.1128/JB.00720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton J.W, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. doi:10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- Hentzer M, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- Hentzer M, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. doi:10.1093/emboj/cdg366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M, Givskov M, Eberl L. Quorum sensing in biofilms: gossip in the slime city. In: Mahmoud G, O'Toole G, editors. Microbial biofilms. ASM Press; Washington, DC: 2004. pp. 118–140. [Google Scholar]
- Hentzer M, Eberl L, Givskov M. Transcriptome analysis of Pseudomonas aeruginosa biofilm development: anaerobic respiration and iron limitation. Biofilms. 2005;2:37–61. doi:10.1017/S1479050505001699 [Google Scholar]
- Heydorn A, Ersboll B, Kato J, Hentzer M, Parsek M.R, Tolker-Nielsen T, Givskov M, Molin S. Statistical analysis of Pseudomonas aeruginosa biofilm development: impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl. Environ. Microbiol. 2002;68:2008–2017. doi: 10.1128/AEM.68.4.2008-2017.2002. doi:10.1128/AEM.68.4.2008-2017.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmgaard T, Persson T, Rasmussen T.B, Givskov M, Nielsen J. Synthesis of furanone-based natural product analogues with quorum sensing antagonist activity. Bioorg. Med. Chem. 2003;11:3261–3271. doi: 10.1016/s0968-0896(03)00295-5. doi:10.1016/S0968-0896(03)00295-5 [DOI] [PubMed] [Google Scholar]
- Hoiby N. Epidemiological investigations of the respiratory tract bacteriology in patients with cystic fibrosis. Acta Pathol. Microbiol. Immunol. Scand. B. 1974;82:541–550. [PubMed] [Google Scholar]
- Hoiby, N. 1977 Pseudomonas aeruginosa infection in cystic fibrosis. Doctoral thesis, University of Copenhagen.
- Howe T.R, Iglewski B.H. Isolation and characterization of alkaline protease-deficient mutants of Pseudomonas aeruginosa in vitro and in a mouse eye model. Infect. Immun. 1984;43:1058–1063. doi: 10.1128/iai.43.3.1058-1063.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.J, Han J.I, Zhang L.H, Leadbetter J.R. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2003;69:5941–5949. doi: 10.1128/AEM.69.10.5941-5949.2003. doi:10.1128/AEM.69.10.5941-5949.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.J, Petersen A, Whiteley M, Leadbetter J.R. Identification of QuiP, the product of gene PA1032, as the second acyl-homoserine lactone acylase of Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2006;72:1190–1197. doi: 10.1128/AEM.72.2.1190-1197.2006. doi:10.1128/AEM.72.2.1190-1197.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya T, Takeoka K, Hiramatsu K, Hirai K, Yamasaki T, Nasu M. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy. 1996;42:186–191. doi: 10.1159/000239440. [DOI] [PubMed] [Google Scholar]
- Kerem B, Rommens J.M, Buchanan J.A, Markiewicz D, Cox T.K, Chakravarti A, Buchwald M, Tsui L.C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. doi:10.1126/science.2570460 [DOI] [PubMed] [Google Scholar]
- Kjelleberg S, Molin S. Is there a role for quorum sensing signals in bacterial biofilms? Curr. Opin. Microbiol. 2002;5:254–258. doi: 10.1016/s1369-5274(02)00325-9. doi:10.1016/S1369-5274(02)00325-9 [DOI] [PubMed] [Google Scholar]
- Kjelleberg S, Steinberg P, Givskov M, Gram L, Manefield M, de Nys R. Do marine natural products interfere with prokeryotic AHL regulatory systems? Aquat. Microb. Ecol. 1997;13:85–93. [Google Scholar]
- Knowles M.R, Boucher R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109:571–577. doi: 10.1172/JCI15217. doi:10.1172/JCI200215217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. Airway biofilms: implications for pathogenesis and therapy of respiratory tract infections. Treat. Respir. Med. 2005;4:241–253. doi: 10.2165/00151829-200504040-00003. doi:10.2165/00151829-200504040-00003 [DOI] [PubMed] [Google Scholar]
- Koch C. Early infection and progression of cystic fibrosis lung disease. Pediatr. Pulmonol. 2002;34:232–236. doi: 10.1002/ppul.10135. doi:10.1002/ppul.10135 [DOI] [PubMed] [Google Scholar]
- Koch C, Hoiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–1069. doi: 10.1016/0140-6736(93)92422-p. doi:10.1016/0140-6736(93)92422-P [DOI] [PubMed] [Google Scholar]
- Koch C, Hoiby N. Diagnosis and treatment of cystic fibrosis. Respiration. 2000;67:239–247. doi: 10.1159/000029503. doi:10.1159/000029503 [DOI] [PubMed] [Google Scholar]
- Konstan M.W, Krenicky J.E, Finney M.R, Kirchner H.L, Hilliard K.A, Hilliard J.B, Davis P.B, Hoppel C.L. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. J. Pharmacol. Exp. Ther. 2003;306:1086–1091. doi: 10.1124/jpet.103.052449. doi:10.1124/jpet.103.052449 [DOI] [PubMed] [Google Scholar]
- Lam J, Chan R, Lam K, Costerton J.W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 1980;28:546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R.N, Dunn W.R, Bycroft B, Camara M, Chhabra S.R, Williams P, Wilson V.G. The Pseudomonas aeruginosa quorum-sensing signal molecule, N-(3-oxododecanoyl)-l-homoserine lactone, inhibits porcine arterial smooth muscle contraction. Br. J. Pharmacol. 1999;128:845–848. doi: 10.1038/sj.bjp.0702870. doi:10.1038/sj.bjp.0702870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manefield M, Turner S.L. Quorum sensing in context: out of molecular biology and into microbial ecology. Microbiology. 2002;148:3762–3764. doi: 10.1099/00221287-148-12-3762. [DOI] [PubMed] [Google Scholar]
- Manefield M, Rasmussen T.B, Henzter M, Andersen J.B, Steinberg P, Kjelleberg S, Givskov M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology. 2002;148:1119–1127. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- Middleton B, Rodgers H.C, Camara M, Knox A.J, Williams P, Hardman A. Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol. Lett. 2002;207:1–7. doi: 10.1111/j.1574-6968.2002.tb11019.x. doi:10.1111/j.1574-6968.2002.tb11019.x [DOI] [PubMed] [Google Scholar]
- Murray P.R, Rosenthal K.S, Kobayashi G.S, Pfaller M.A. 4th edn. Mosby; St. Louis, MO: 2002. Medical microbiology. [Google Scholar]
- National Institutes of Health 2004 The problem of antibiotic resistance. See http://www.niaid.nih.gov/factsheets/antimicro.htm
- Nicas T.I, Frank D.W, Stenzel P, Lile J.D, Iglewski B.H. Role of exoenzyme S in chronic Pseudomonas aeruginosa lung infections. Eur. J. Clin. Microbiol. 1985;4:175–179. doi: 10.1007/BF02013593. doi:10.1007/BF02013593 [DOI] [PubMed] [Google Scholar]
- Nilsson P, Olofsson A, Fagerlind M, Fagerstrom T, Rice S, Kjelleberg S, Steinberg P. Kinetics of the AHL regulatory system in a model biofilm system: how many bacteria constitute a “quorum”? J. Mol. Biol. 2001;309:631–640. doi: 10.1006/jmbi.2001.4697. doi:10.1006/jmbi.2001.4697 [DOI] [PubMed] [Google Scholar]
- Norrby S.R, Nord C.E, Finch R. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect. Dis. 2005;5:115–119. doi: 10.1016/S1473-3099(05)01283-1. [DOI] [PubMed] [Google Scholar]
- Pearson J.P, Pesci E.C, Iglewski B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.P, Feldman M, Iglewski B.H, Prince A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 2000;68:4331–4334. doi: 10.1128/iai.68.7.4331-4334.2000. doi:10.1128/IAI.68.7.4331-4334.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S.S, Hoiby N, Espersen F, Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax. 1992;47:6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci E.C, Pearson J.P, Seed P.C, Iglewski B.H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts S.B, Roggli V.L, Spock A. Immunohistologic quantification of Pseudomonas aeruginosa in the tracheobronchial tree from patients with cystic fibrosis. Pediatr. Pathol. Lab. Med. 1995;15:707–721. doi: 10.3109/15513819509027007. [DOI] [PubMed] [Google Scholar]
- Preston M.J, Seed P.C, Toder D.S, Iglewski B.H, Ohman D.E, Gustin J.K, Goldberg J.B, Pier G.B. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect. Immun. 1997;65:3086–3090. doi: 10.1128/iai.65.8.3086-3090.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch M, et al. An inhibitor of bacterial quorum sensing reduces mortalities caused by vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum) Syst. Appl. Microbiol. 2004;27:350–359. doi: 10.1078/0723-2020-00268. doi:10.1078/0723-2020-00268 [DOI] [PubMed] [Google Scholar]
- Rasmussen T.B, Bjarnsholt T, Skindersoe M.E, Hentzer M, Kristoffersen P, Kote M, Eberl L, Nielsen J, Givskov M. Screening for quorum sensing inhibitors using a novel genetic system—the QSI selector. J. Bacteriol. 2005a;187:1799–1814. doi: 10.1128/JB.187.5.1799-1814.2005. doi:10.1128/JB.187.5.1799-1814.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T.B, Skindersoe M.E, Bjarnsholt T, Christensen K.B, Andersen J.B, Ostenfeld-Larsen T, Hentzer M, Givskov M. Identity and effects of quorum sensing inhibitors produced by Penicillum species. Microbiology. 2005b;151:1325–1340. doi: 10.1099/mic.0.27715-0. doi:10.1099/mic.0.27715-0 [DOI] [PubMed] [Google Scholar]
- Riordan J.R, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. doi:10.1126/science.2475911 [DOI] [PubMed] [Google Scholar]
- Rommens J.M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. doi:10.1126/science.2772657 [DOI] [PubMed] [Google Scholar]
- Rubenstein R.C. Novel, mechanism-based therapies for cystic fibrosis. Curr. Opin. Pediatr. 2005;17:385–392. doi: 10.1097/01.mop.0000158846.95469.6f. doi:10.1097/01.mop.0000158846.95469.6f [DOI] [PubMed] [Google Scholar]
- Rumbaugh K.P, Griswold J.A, Iglewski B.H, Hamood A.N. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 1999;67:5854–5862. doi: 10.1128/iai.67.11.5854-5862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh K.P, Griswold J.A, Hamood A.N. The role of quorum sensing in the in vivo virulence of Pseudomonas aeruginosa. Microbes Infect. 2000;2:1721–1731. doi: 10.1016/s1286-4579(00)01327-7. doi:10.1016/S1286-4579(00)01327-7 [DOI] [PubMed] [Google Scholar]
- Saiman L. Microbiology of early CF lung disease. Paediatr. Respir. Rev. 2004a;5:S367–S369. doi: 10.1016/s1526-0542(04)90065-6. doi:10.1016/S1526-0542(04)90065-6 [DOI] [PubMed] [Google Scholar]
- Saiman L. The use of macrolide antibiotics in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 2004b;10:515–523. doi: 10.1097/01.mcp.0000142101.53084.f0. doi:10.1097/01.mcp.0000142101.53084.f0 [DOI] [PubMed] [Google Scholar]
- Schuster M, Lostroh C.P, Ogi T, Greenberg E.P. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. doi:10.1128/JB.185.7.2066-2079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.K, Schaefer A.L, Parsek M.R, Moninger T.O, Welsh M.J, Greenberg E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. doi:10.1038/35037627 [DOI] [PubMed] [Google Scholar]
- Southern K.W, Barker P.M. Azithromycin for cystic fibrosis. Eur. Respir. J. 2004;24:834–838. doi: 10.1183/09031936.04.00084304. doi:10.1183/09031936.04.00084304 [DOI] [PubMed] [Google Scholar]
- Storey D.G, Ujack E.E, Rabin H.R, Mitchell I. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect. Immun. 1998;66:2521–2528. doi: 10.1128/iai.66.6.2521-2528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C.K, et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. doi:10.1038/35023079 [DOI] [PubMed] [Google Scholar]
- Taga M.E, Bassler B.L. Chemical communication among bacteria. Proc. Natl Acad. Sci. USA. 2003;100:14 549–14 554. doi: 10.1073/pnas.1934514100. doi:10.1073/pnas.1934514100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Kays M, Prince A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect. Immun. 1995;63:1278–1285. doi: 10.1128/iai.63.4.1278-1285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H.B, DiMango E, Bryan R, Gambello M, Iglewski B.H, Goldberg J.B, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect. Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda K, Comte R, Pechere J.C, Kohler T, Yamaguchi K, van Delden C. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2001;45:1930–1933. doi: 10.1128/AAC.45.6.1930-1933.2001. doi:10.1128/AAC.45.6.1930-1933.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delden C, Iglewski B.H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delden C, Comte R, Bally A.M. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 2001;183:5376–5384. doi: 10.1128/JB.183.18.5376-5384.2001. doi:10.1128/JB.183.18.5376-5384.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M.L, Grant C.C, Prince R.W. Regulation of exotoxin A synthesis in Pseudomonas aeruginosa: characterization of toxA–lacZ fusions in wild-type and mutant strains. Mol. Microbiol. 1989;3:371–381. doi: 10.1111/j.1365-2958.1989.tb00182.x. doi:10.1111/j.1365-2958.1989.tb00182.x [DOI] [PubMed] [Google Scholar]
- Vasil M.L, Graham L.M, Ostroff R.M, Shortridge V.D, Vasil A.I. Phospholipase C: molecular biology and contribution to the pathogenesis of Pseudomonas aeruginosa. Antibiot. Chemother. 1991;44:34–47. doi: 10.1159/000420295. [DOI] [PubMed] [Google Scholar]
- Wagner V.E, Bushnell D, Passador L, Brooks A.I, Iglewski B.H. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 2003;185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. doi:10.1128/JB.185.7.2080-2095.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P, et al. Quorum sensing and the population-dependent control of virulence. Phil. Trans. R. Soc. B. 2000;355:667–680. doi: 10.1098/rstb.2000.0607. doi:10.1098/rstb.2000.0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P, Winzer K, Chan W, Cámara M. Look who's talking: communication and quorum sensing in the bacterial world. Phil. Trans. R. Soc. B. 2007;362:1119–1134. doi: 10.1098/rstb.2007.2039. doi:10.1098/rstb.2007.2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D.E, Cryz S.J, Friedman R.L, Iglewski B.H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect. Immun. 1982;36:1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzsch D, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 2002;109:317–325. doi: 10.1172/JCI13870. doi:10.1172/JCI200213870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzsch D, Ulrich M, Bragonzi A, Viglio S, Weidemann B, Doring G. Alveolar tissue destruction by macrophages and neutrophils in cystic fibrosis. Pediatr. Pulmonol. 2004;27(Suppl.):277. Abstract. [Google Scholar]
- Wu H, et al. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology. 2000;146:2481–2493. doi: 10.1099/00221287-146-10-2481. [DOI] [PubMed] [Google Scholar]
- Wu H, Song Z, Givskov M, Doring G, Worlitzsch D, Mathee K, Rygaard J, Hoiby N. Pseudomonas aeruginosa mutations in lasI and rhlI quorum sensing systems result in milder chronic lung infection. Microbiology. 2001;147:1105–1113. doi: 10.1099/00221287-147-5-1105. [DOI] [PubMed] [Google Scholar]
- Wu H, Song Z, Givskov M, Hoiby N. Effects of quorum-sensing on immunoglobulin G responses in a rat model of chronic lung infection with Pseudomonas aeruginosa. Microbes Infect. 2004a;6:34–37. doi: 10.1016/j.micinf.2003.10.006. doi:10.1016/j.micinf.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Wu H, Song Z, Hentzer M, Andersen J.B, Molin S, Givskov M, Hoiby N. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 2004b;53:1054–1061. doi: 10.1093/jac/dkh223. doi:10.1093/jac/dkh223 [DOI] [PubMed] [Google Scholar]