Abstract
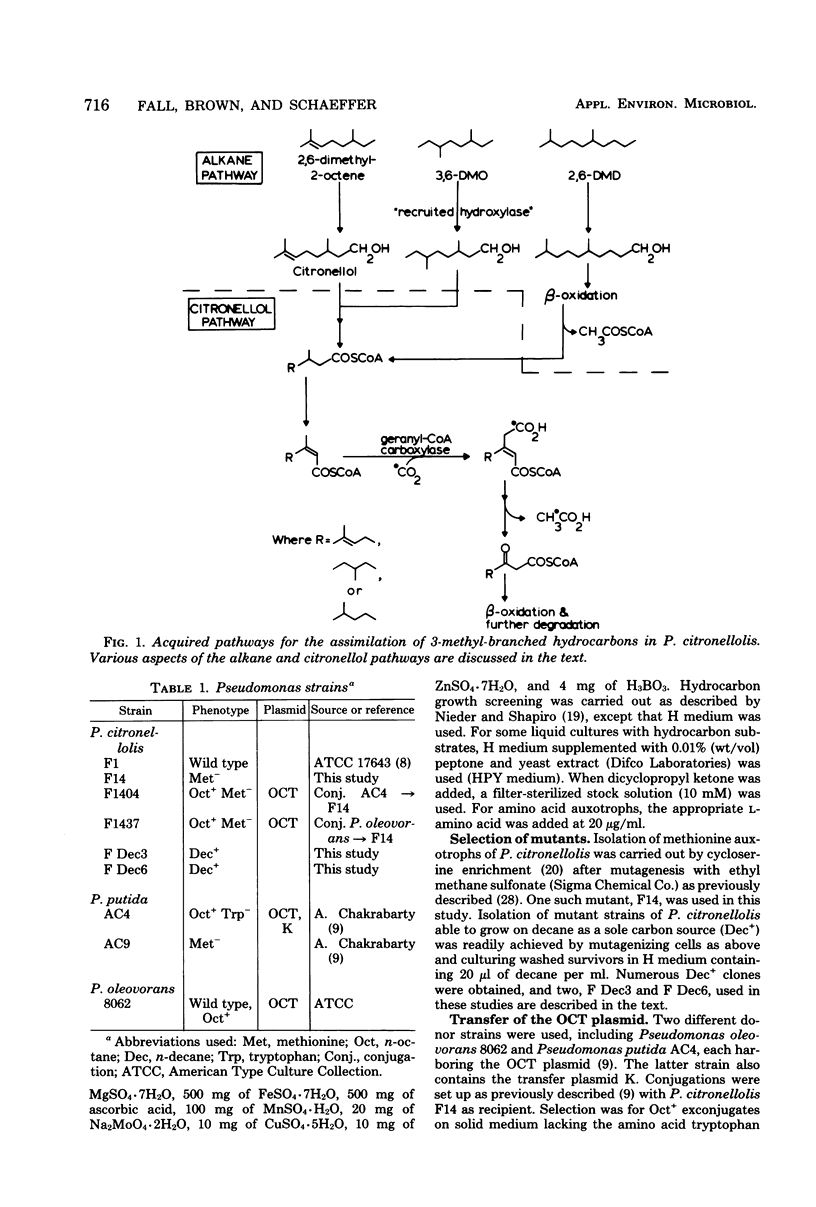
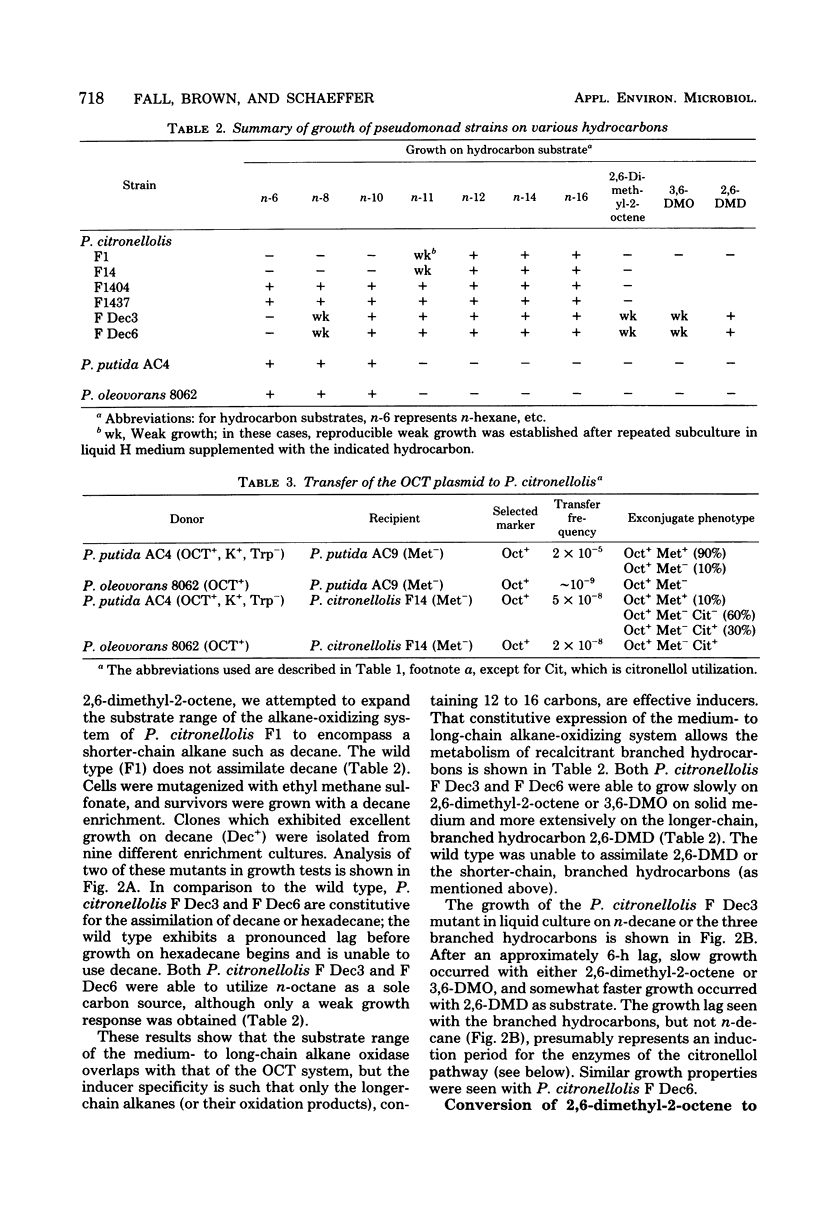
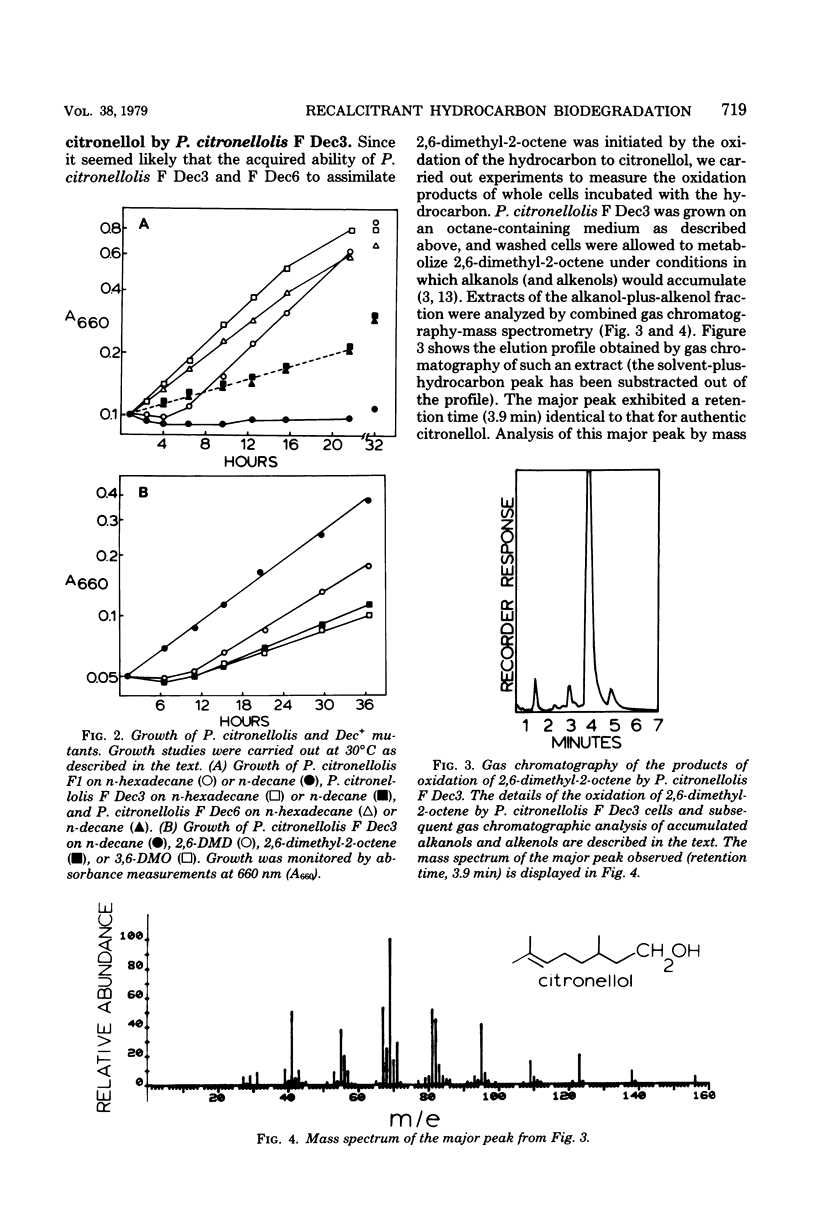
Experiments were carried out to construct pseudomonad strains capable of the biodegradation of certain recalcitrant branched hydrocarbons via a combination of alkane and citronellol degradative pathways. To promote the metabolism of the recalcitrant hydrocarbon 2,6-dimethyl-2-octene we transferred the OCT plasmid to Pseudomonas citronellolis, a pseudomonad containing the citronellol pathway. This extended the n-alkane substrate range of the organism, but did not permit utilization of the branched hydrocarbon even in the presence of a gratuitous inducer of the OCT plasmid. In a separate approach n-decane-utilizing (Dec+) mutants of P. citronellolis were selected and found to be constitutive for the expression of medium- to long-chain alkane oxidation. The Dec+ mutants were capable of degradation of 2,6-dimethyl-2-octene via the citronellol pathway as shown by (i) conversion of the hydrocarbon to citronellol, determined by gas-liquid chromatography-mass spectrometry, (ii) induction of geranyl-coenzyme A carboxylase, a key enzyme of the citronellol pathway, and (iii) demonstration of beta-decarboxymethylation of the hydrocarbon by whole cells. The Dec+ mutants had also acquired the capacity to metabolize other recalcitrant branched hydrocarbons such as 3,6-dimethyloctane and 2,6-dimethyldecane. These studies demonstrate how enzyme recruitment can provide a pathway for the biodegradation of otherwise recalcitrant branched hydrocarbons.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZOULAY E., HEYDEMAN M. T. Extraction and properties of alcohol dehydrogenase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1963 May 7;73:1–6. doi: 10.1016/0006-3002(63)90353-6. [DOI] [PubMed] [Google Scholar]
- Bartha R. The microbiology of aquatic oil spills. Adv Appl Microbiol. 1977;22:225–266. doi: 10.1016/s0065-2164(08)70164-3. [DOI] [PubMed] [Google Scholar]
- Benson S., Fennewald M., Shapiro J., Huettner C. Fractionation of inducible alkane hydroxylase activity in Pseudomonas putida and characterization of hydroxylase-negative plasmid mutations. J Bacteriol. 1977 Nov;132(2):614–621. doi: 10.1128/jb.132.2.614-621.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Shapiro J. Induction of alkane hydroxylase proteins by unoxidized alkane in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):759–760. doi: 10.1128/jb.123.2.759-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Shapiro J. Plasmid-determined alcohol dehydrogenase activity in alkane-utilizing strains of Pseudomonas putida. J Bacteriol. 1976 May;126(2):794–798. doi: 10.1128/jb.126.2.794-798.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell S. G., Lau E. P., Watt D. S., Fall R. R. Biodegradation of acyclic isoprenoids by Pseudomonas species. J Bacteriol. 1978 Aug;135(2):324–333. doi: 10.1128/jb.135.2.324-333.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Chou G., Gunsalus I. C. Genetic regulation of octane dissimilation plasmid in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1137–1140. doi: 10.1073/pnas.70.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Dissociation of a degradative plasmid aggregate in Pseudomonas. J Bacteriol. 1974 Jun;118(3):815–820. doi: 10.1128/jb.118.3.815-820.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Grund A., Shapiro J., Fennewald M., Bacha P., Leahy J., Markbreiter K., Nieder M., Toepfer M. Regulation of alkane oxidation in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):546–556. doi: 10.1128/jb.123.2.546-556.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Utilization of aliphatic hydrocarbons by micro-organisms. Adv Microb Physiol. 1971;5:1–43. doi: 10.1016/s0065-2911(08)60404-x. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Putnam S. L. Sensitive biuret method for determination of protein in an impure system such as whole bacteria. Anal Biochem. 1971 Nov;44(1):239–245. doi: 10.1016/0003-2697(71)90366-6. [DOI] [PubMed] [Google Scholar]
- McKenna E. J., Coon M. J. Enzymatic omega-oxidation. IV. Purification and properties of the omega-hydroxylase of Pseudomonas oleovorans. J Biol Chem. 1970 Aug 10;245(15):3882–3889. [PubMed] [Google Scholar]
- McKenna E. J., Kallio R. E. The biology of hydrocarbons. Annu Rev Microbiol. 1965;19:183–208. doi: 10.1146/annurev.mi.19.100165.001151. [DOI] [PubMed] [Google Scholar]
- Nieder M., Shapiro J. Physiological function of the Pseudomonas putida PpG6 (Pseudomonas oleovorans) alkane hydroxylase: monoterminal oxidation of alkanes and fatty acids. J Bacteriol. 1975 Apr;122(1):93–98. doi: 10.1128/jb.122.1.93-98.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Ornston M. K., Chou G. Isolation of spontaneous mutant strains of Pseudomonas putida. Biochem Biophys Res Commun. 1969 Jul 7;36(1):179–184. doi: 10.1016/0006-291x(69)90666-4. [DOI] [PubMed] [Google Scholar]
- Pirnik M. P. Microbial oxidation of methyl branched alkanes. CRC Crit Rev Microbiol. 1977 Sep;5(4):413–422. doi: 10.3109/10408417709102812. [DOI] [PubMed] [Google Scholar]
- SEUBERT W., FASS E. UNTERSUCHUNGEN UEBER DEN BAKTERIELLEN ABBAU VON ISOPRENOIDEN. V. DER MECHANISMUS DES ISOPRENOIDABBAUES. Biochem Z. 1964 Dec 7;341:35–44. [PubMed] [Google Scholar]
- SEUBERT W., REMBERGER U. UNTERSUCHUNGEN UEBER DEN BAKTERIELLEN ABBAU VON ISOPRENOIDEN. II. DIE ROLLE DER KOHLENSAEURE. Biochem Z. 1963;338:245–264. [PubMed] [Google Scholar]
- Schaeffer T. L., Cantwell S. G., Brown J. L., Watt D. S., Fall R. R. Microbial growth on hydrocarbons: terminal branching inhibits biodegradation. Appl Environ Microbiol. 1979 Oct;38(4):742–746. doi: 10.1128/aem.38.4.742-746.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. M., Holloway B. W. Suppressor mutations in Pseudomonas aeruginosa. J Bacteriol. 1976 Mar;125(3):780–786. doi: 10.1128/jb.125.3.780-786.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eyk J., Bartels T. J. Paraffin oxidation in Pseudomonas aeruginosa. I. Induction of paraffin oxidation. J Bacteriol. 1968 Sep;96(3):706–712. doi: 10.1128/jb.96.3.706-712.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden A. C., Thijsse G. J. The mechanisms of microbial oxidations of petroleum hydrocarbons. Adv Enzymol Relat Areas Mol Biol. 1965;27:469–546. doi: 10.1002/9780470122723.ch10. [DOI] [PubMed] [Google Scholar]


