Abstract
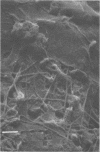
A project to investigate biofouling, under conditions relevant to ocean thermal energy conversion heat exchangers, was conducted during July through September 1977 at a site about 13 km north of St. Croix (U.S. Virgin Islands). Seawater was drawn from a depth of 20 m, within the surface mixed layer, through aluminum pipes (2.6 m long, 2.5-cm internal diameter) at flow velocities of about 0.9 and 1.8 m/s. The temperature of the seawater entering the mock heat exchanger units was between 27.8 and 28.6°C. After about 10 weeks of exposure to seawater, when their thermal conductivity was reported to be significantly impaired, the pipes were assayed for the accumulation of biological material on their inner surfaces. The extent of biofouling was very low and independent of flow velocity. Bacterial populations, determined from plate counts, were about 107 cells per cm2. The ranges of mean areal densities for other biological components were: organic carbon, 18 to 27 μg/cm2; organic nitrogen, 1.5 to 3.0 μg/cm2; adenosine 5′-triphosphate, 4 to 28 ng/cm2; carbohydrate (as glucose in the phenol assay), 3.8 to 7.0 μg/cm2; chlorophyll a, 0.2 to 0.8 ng/cm2. It was estimated from the adenosine 5′-triphosphate and nitrogen contents that the layer of live bacteria present after 10 weeks was only of the order of 1μm thick. The C/N ratio of the biological material suggested the presence of extracellular polysaccharidic material. Such compounds, because of their water-retaining capacities, could account for the related increase in thermal resistance associated with the pipes. This possibility merits further investigation, but the current results emphasize the minor degree of biofouling which is likely to be permissible in ocean thermal energy conversion heat exchangers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cagle G. D. Fine structure and distribution of extracellular polymer surrounding selected aerobic bacteria. Can J Microbiol. 1975 Mar;21(3):395–408. doi: 10.1139/m75-055. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Motta E. J. Kinetics of growth and substrate uptake in a biological film system. Appl Environ Microbiol. 1976 Feb;31(2):286–293. doi: 10.1128/aem.31.2.286-293.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis R. R., Brown O. R. ATP concentration in Escherichia coli during oxygen toxicity. Biochim Biophys Acta. 1976 Sep 13;440(3):723–732. doi: 10.1016/0005-2728(76)90054-2. [DOI] [PubMed] [Google Scholar]
- Metz W. D. Ocean thermal energy: the biggest gamble in solar power. Science. 1977 Oct 14;198(4313):178–180. doi: 10.1126/science.198.4313.178. [DOI] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]