Abstract
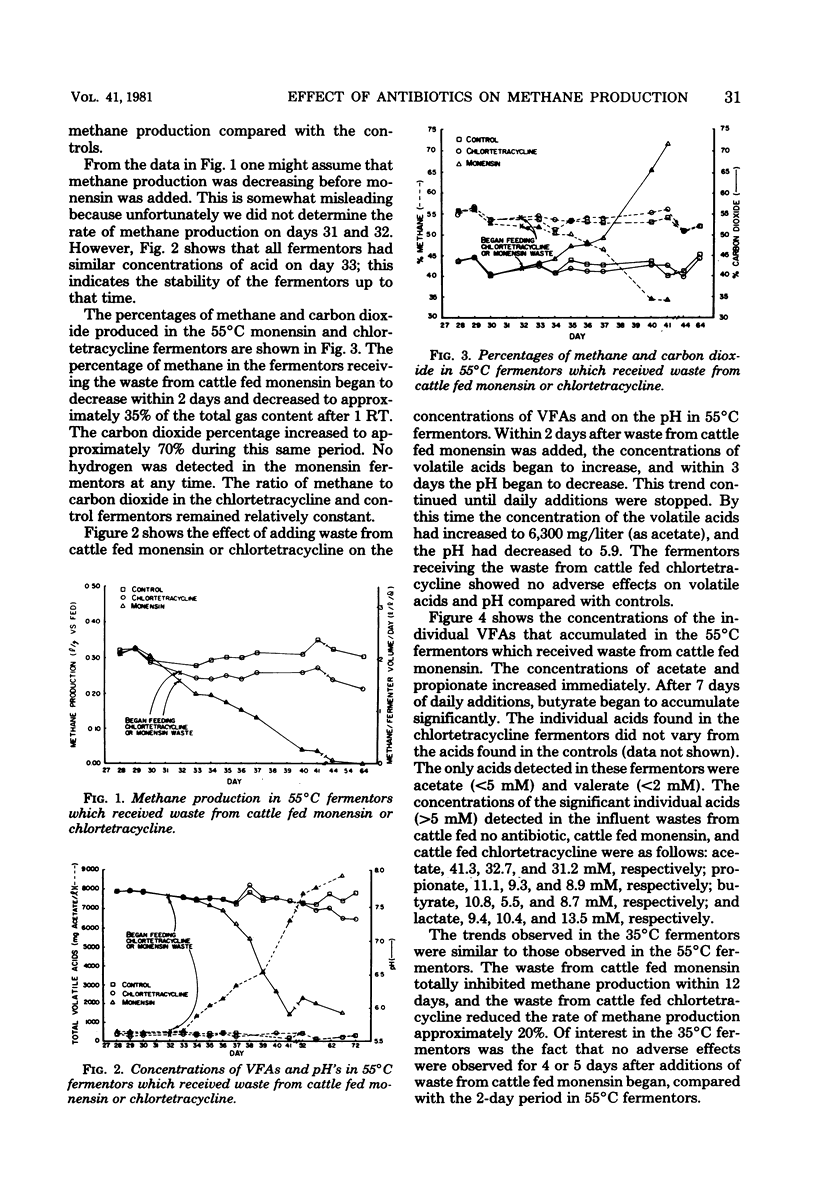
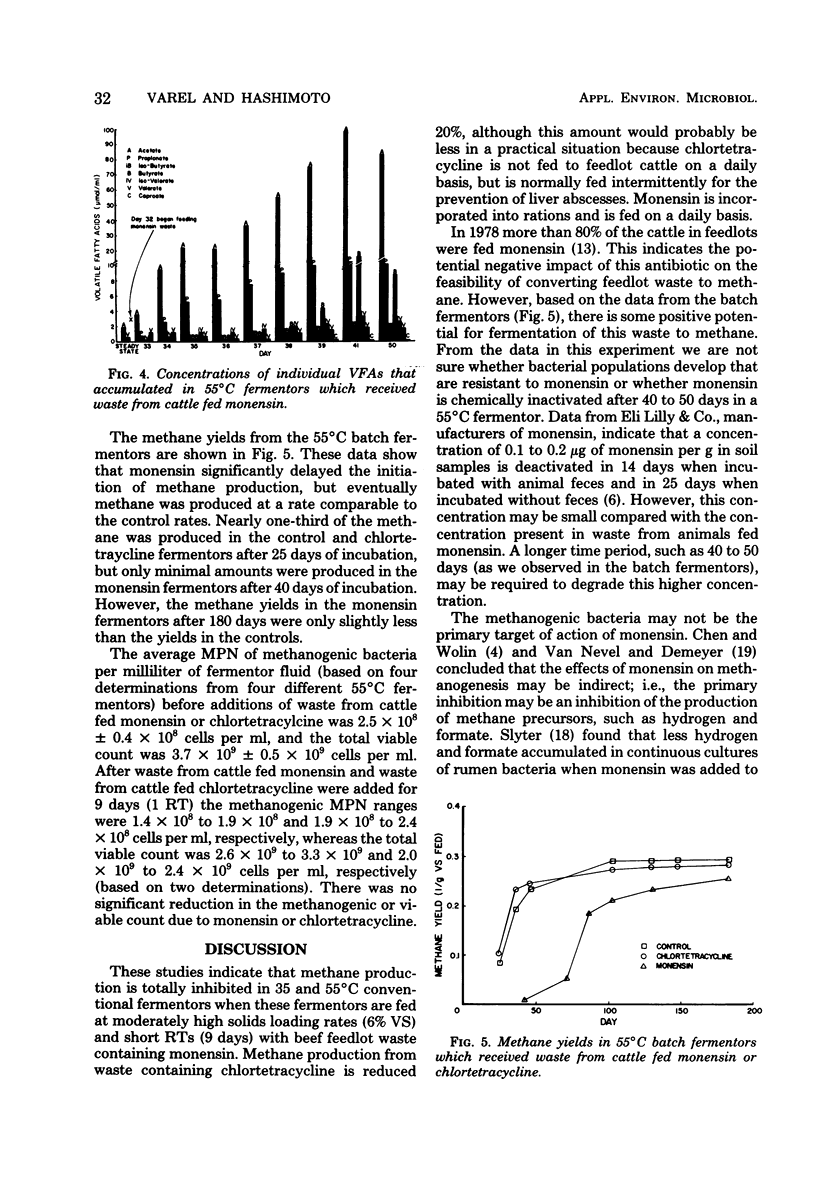
Wastes from feedlot cattle fed finishing diets containing either monensin, chlortetracycline, or no antibiotic were investigated as substrates for methane production. We used continuously mixed anaerobic fermentors with 3-liter working volumes at 35 and 55°C; these fermentors were fed once per day. Within a few days after waste from animals fed monensin was added, the volume of methane produced began to decrease in the 55°C fermentors. After 9 days of daily feeding, methane production was severely inhibited, the pH dropped from 7.6 to 5.9, and the concentration of volatile acids increased from 543 to 6,300 mg/liter (as acetate). Although additions of waste from cattle fed monensin were discontinued after 9 days, the fermentors did not resume gas production within 8 weeks. The addition of waste from cattle which had been fed chlortetracycline reduced the methane production rate approximately 20%; however, pH and volatile acid values were comparable to control fermentor values after 40 days. Similar effects were observed with the 35°C fermentors. In a batch fermentation experiment in which 50-g portions of volatile solids from waste of animals fed monensin, chlortetracycline, or no antibiotics were added to fermentors, monensin delayed the onset of methane production for about 40 days, but then these fermentors began to produce methane at a rate comparable to the control rate. The ultimate methane yields from the three types of waste after 180 days were not significantly different. These studies indicate that monensin has a detrimental effect on the conversion of feedlot wastes to methane, unless microorganisms can be adapted to the levels that are present in these wastes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho A., Manthey J., Occolowitz J., Zornes L. Metabolism of monensin in the steer and rat. J Agric Food Chem. 1978 Sep-Oct;26(5):1090–1095. doi: 10.1021/jf60219a005. [DOI] [PubMed] [Google Scholar]
- Hauser K. J., Zabransky R. J. Modification of the gas-liquid chromatography procedure and evaluation of a new column packing material for the identification of anaerobic bacteria. J Clin Microbiol. 1975 Jul;2(1):1–7. doi: 10.1128/jcm.2.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg R., Manthey J., Richardson L., Cooley C., Donoho A. Excretion and tissue distribution of [14C]monensin in cattle. J Agric Food Chem. 1978 Sep-Oct;26(5):1087–1090. doi: 10.1021/jf60219a004. [DOI] [PubMed] [Google Scholar]
- Salanitro J. P., Fairchilds I. G., Zgornicki Y. D. Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Appl Microbiol. 1974 Apr;27(4):678–687. doi: 10.1128/am.27.4.678-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyter L. L. Monensin and dichloroacetamide influences on methane and volatile Fatty Acid production by rumen bacteria in vitro. Appl Environ Microbiol. 1979 Feb;37(2):283–288. doi: 10.1128/aem.37.2.283-288.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nevel C. J., Demeyer D. I. Effect of monensin on rumen metabolism in vitro. Appl Environ Microbiol. 1977 Sep;34(3):251–257. doi: 10.1128/aem.34.3.251-257.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Hashimoto A. G., Chen Y. R. Effect of temperature and retention time on methane production from beef cattle waste. Appl Environ Microbiol. 1980 Aug;40(2):217–222. doi: 10.1128/aem.40.2.217-222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Isaacson H. R., Bryant M. P. Thermophilic methane production from cattle waste. Appl Environ Microbiol. 1977 Feb;33(2):298–307. doi: 10.1128/aem.33.2.298-307.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


