Abstract
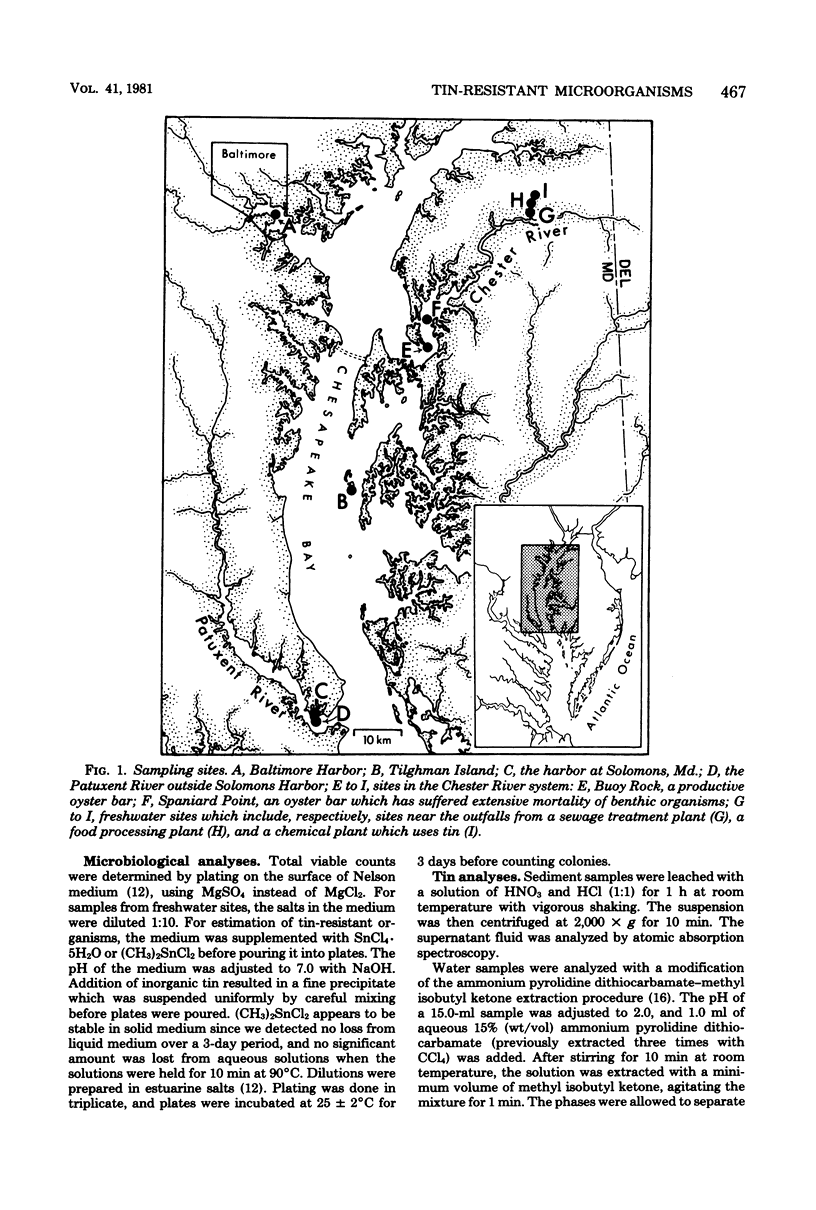
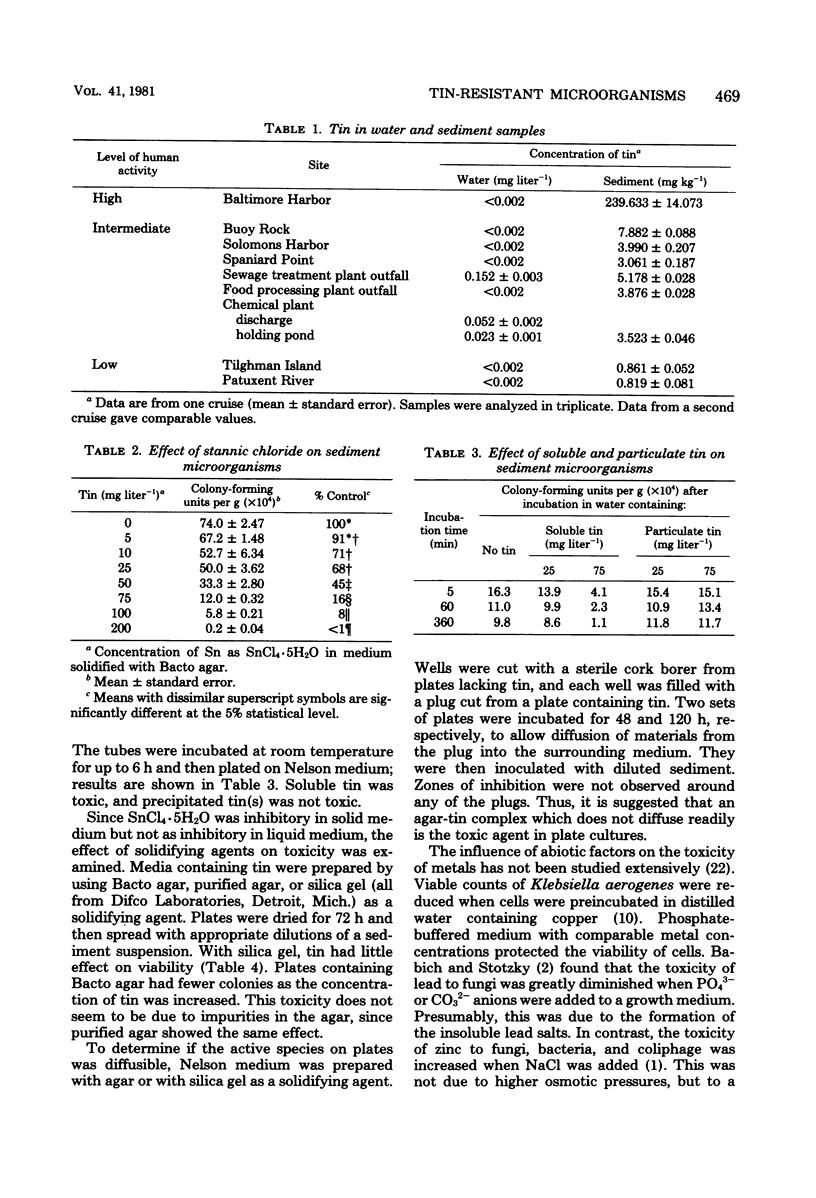
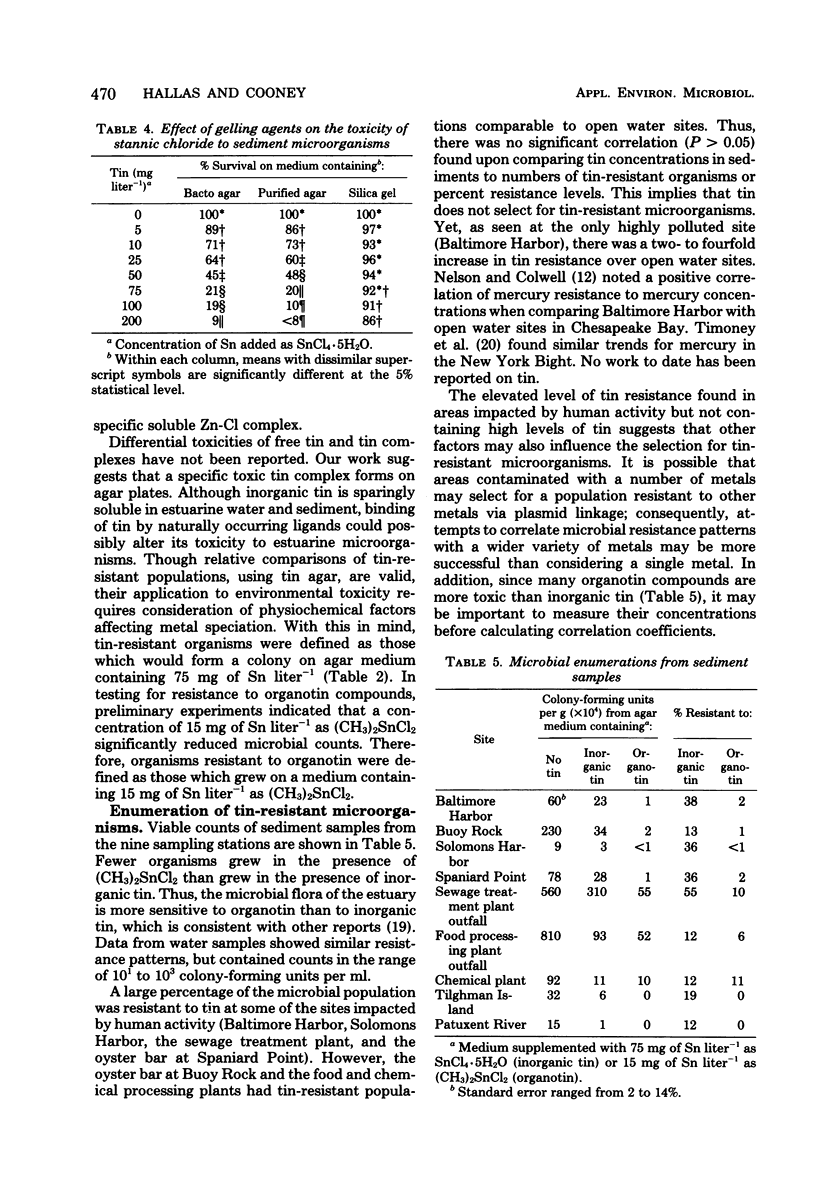
Sediment and water samples from nine stations in Chesapeake Bay were examined for tin content and for microbial populations resistant to inorganic tin (75 mg of Sn liter−1 as SnCl4·5H2O) or to the organotin compound dimethyltin chloride [15 mg of Sn liter−1 as (CH3)2SnCl2]. Tin concentrations in sediments were higher (3.0 to 7.9 mg kg−1) at sites impacted by human activity than at open water sites (0.8 to 0.9 mg kg−1), and they were very high (239.6 mg kg−1) in Baltimore Harbor, which is impacted by both shipping and heavy industry. Inorganic tin (75 mg Sn liter−1) in agar medium significantly decreased viable counts, but its toxicity was markedly reduced in liquid medium; it was not toxic in medium solidified with silica gel. Addition of SnCl4·5H2O to these media produced a tin precipitate which was not involved in the metal's toxicity. The data suggest that a soluble tin-agar complex which is toxic to cells is formed in agar medium. Thus, the toxicity of tin depends more on the chemical species than on the metal concentration in the medium. All sites in Chesapeake Bay contained organisms resistant to tin. The microbial flora was more sensitive to (CH3)2SnCl2 than to SnCl4·5H2O. The elevated level of tin-resistant microorganisms in some aeas not containing unusually high tin concentrations suggests that factors other than tin may participate in the selection for a tin-tolerant microbial flora.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich H., Stotzky G. Abiotic factors affecting the toxicity of lead to fungi. Appl Environ Microbiol. 1979 Sep;38(3):506–513. doi: 10.1128/aem.38.3.506-513.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich H., Stotzky G. Toxicity of zinc to fungi, bacteria, and coliphages: influence of chloride ions. Appl Environ Microbiol. 1978 Dec;36(6):906–914. doi: 10.1128/aem.36.6.906-914.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braman R. S., Tompkins M. A. Separation and determination of nanogram amounts of inorganic tin and methyltin compounds in the environment. Anal Chem. 1979 Jan;51(1):12–19. doi: 10.1021/ac50037a011. [DOI] [PubMed] [Google Scholar]
- DAUM R. J. AGRICULTURAL AND BIOCIDAL APPLICATIONS OF ORGANOMETALLICS. Ann N Y Acad Sci. 1965 May 28;125:229–241. doi: 10.1111/j.1749-6632.1965.tb45393.x. [DOI] [PubMed] [Google Scholar]
- Holden A. V. The effects of pesticides on life in fresh waters. Proc R Soc Lond B Biol Sci. 1972 Mar 21;180(1061):383–394. doi: 10.1098/rspb.1972.0025. [DOI] [PubMed] [Google Scholar]
- MacLeod R. A., Kuo S. C., Gelinas R. Metabolic injury to bacteria. II. Metabolic injury induced by distilled water or Cu++ in the plating diluent. J Bacteriol. 1967 Mar;93(3):961–969. doi: 10.1128/jb.93.3.961-969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A. L., Colwell R. R. Microbiological effects of metal ions in Chesapeake Bay water and sediment. Bull Environ Contam Toxicol. 1977 Jul;18(1):99–103. doi: 10.1007/BF01686313. [DOI] [PubMed] [Google Scholar]
- Timoney J. F., Port J., Giles J., Spanier J. Heavy-metal and antibiotic resistance in the bacterial flora of sediments of New York Bight. Appl Environ Microbiol. 1978 Sep;36(3):465–472. doi: 10.1128/aem.36.3.465-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


