Abstract
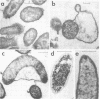
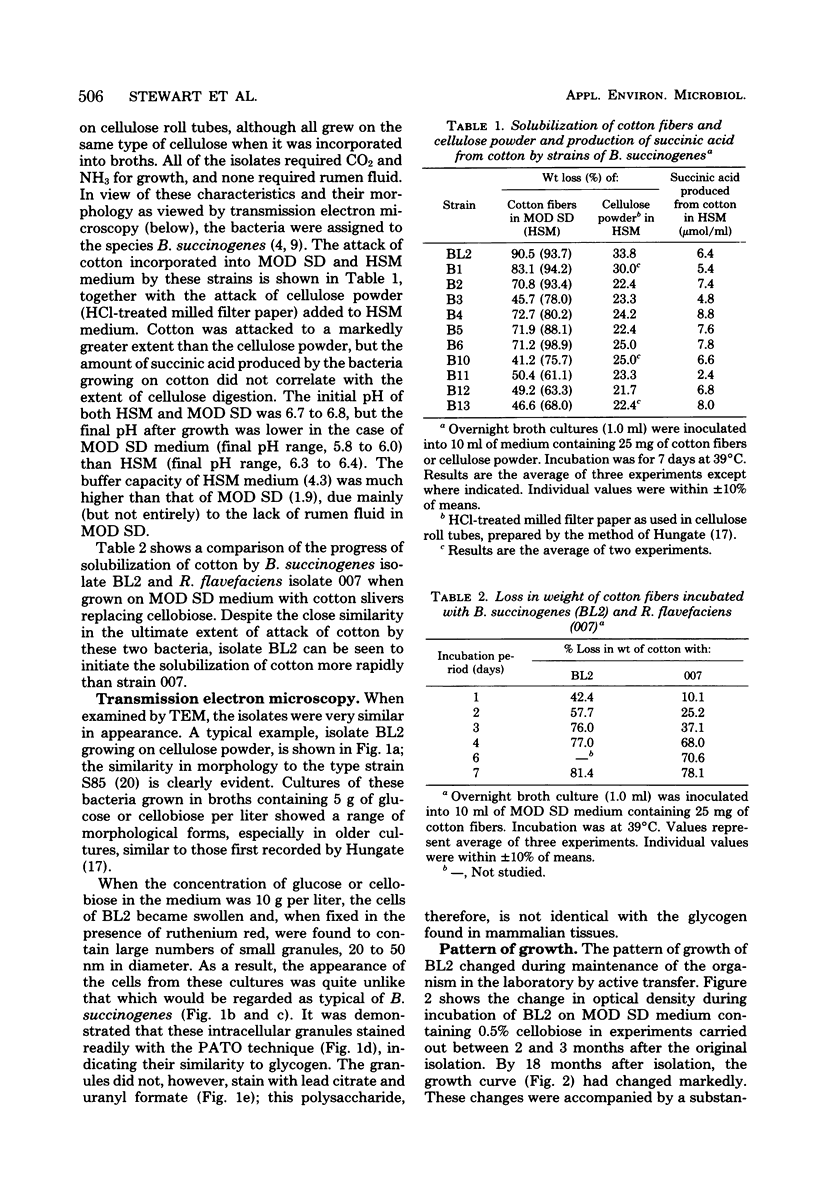
Eleven isolates of Bacteriodes succinogenes were obtained from the rumen of a cow by an enrichment method with dewaxed cotton fibers as the selective substrate. All of the isolates degraded cotton fibers, but none formed clear zones in cellulose agar, having only a limited ability to degrade the type of cellulose powder used. One isolate, BL2, was studied in greater detail and was found to accumulate a glycogen-like polysaccharide when excess (0.5 to 1.0%) soluble carbohydrate was supplied in the nutrient medium. Although the pattern of growth and polysaccharide accumulation by strain BL2 changed during maintenance of the organism in the laboratory, the maximum amount of carbohydrate found in the cells was constant, at around 74% of the cell dry weight. The findings are discussed in relation to the methods of assessing the role of B. succinogenes in the rumen fermentation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Brown R. G., Costerton J. W. Characterization of a Cytoplasmic Reserve Glucan from Ruminococcus albus. Appl Environ Microbiol. 1977 Mar;33(3):718–724. doi: 10.1128/aem.33.3.718-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Alkaline phosphatase activity of rumen bacteria. Appl Environ Microbiol. 1977 Nov;34(5):586–590. doi: 10.1128/aem.34.5.586-590.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings G. F., Yokoyama M. T. Gas-liquid chromatography for evaluating polysaccharide degradation by Ruminococcus flavefaciens C94 and Bacteroides succinogenes S85. Appl Environ Microbiol. 1980 Mar;39(3):566–571. doi: 10.1128/aem.39.3.566-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale D., Morris E. J., Bacon J. S. Electron microscopy of the microbial populations present and their modes of attack on various cellulosic substrates undergoing digestion in the sheep rumen. Appl Environ Microbiol. 1978 Jul;36(1):160–168. doi: 10.1128/aem.36.1.160-168.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL G., BRYANT M. P. THE CELLULOLYTIC ACTIVITY OF PURE STRAINS OF BACTERIA FROM THE RUMEN OF CATTLE. J Gen Microbiol. 1963 Sep;32:441–448. doi: 10.1099/00221287-32-3-441. [DOI] [PubMed] [Google Scholar]
- HANKER J. S., SEAMAN A. R., WEISS L. P., UENO H., BERGMAN R. A., SELIGMAN A. M. OSMIOPHILIC REAGENTS: NEW CYTOCHEMICAL PRINCIPLE FOR LIGHT AND ELECTRON MICROSCOPY. Science. 1964 Nov 20;146(3647):1039–1043. doi: 10.1126/science.146.3647.1039. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B. D., Henderson C., Asmundson R. V. The role of carbonate in the metabolism of glucose by Butyrivibrio fibrisolvens. J Gen Microbiol. 1978 Apr;105(2):287–295. doi: 10.1099/00221287-105-2-287. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Sharpe M. E., Sutton J. D. The microbial flora of the rumen of cows fed hay and high cereal rations and its relationship to the rumen fermentation. J Appl Bacteriol. 1971 Jun;34(2):425–434. doi: 10.1111/j.1365-2672.1971.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Lindner J. G., Marcelis J. H., de Vos N. M., Hoogkamp-Korstanje J. A. Intracellular polysaccharide of Bacteroides fragilis. J Gen Microbiol. 1979 Mar;111(1):93–99. doi: 10.1099/00221287-111-1-93. [DOI] [PubMed] [Google Scholar]
- MARGHERITA S. S., HUNGATE R. E., STORZ H. VARIATION IN RUMEN BUTYRIVIBRIO STRAINS. J Bacteriol. 1964 Jun;87:1304–1308. doi: 10.1128/jb.87.6.1304-1308.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. O., Stewart C. S. Establishment of a limited rumen flora in gnotobiotic lambs fed on a roughage diet. J Gen Microbiol. 1974 Oct;84(2):379–382. doi: 10.1099/00221287-84-2-379. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane B. S., Gouws L., Kistner A. Cellulolytic bacteria occurring in the rumen of sheep conditioned to low-protein teff hay. J Gen Microbiol. 1969 Mar;55(3):445–457. doi: 10.1099/00221287-55-3-445. [DOI] [PubMed] [Google Scholar]
- Stewart C. S. Factors affecting the cellulolytic activity of rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):497–502. doi: 10.1128/aem.33.3.497-502.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J. Cytoplasmic reserve polysaccharide of Selenomonas ruminantium. Appl Environ Microbiol. 1980 Mar;39(3):630–634. doi: 10.1128/aem.39.3.630-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]