Abstract
Genetic modification of Serratia marcescens QMB1466 was undertaken to isolated mutants which produce increased levels of chitinolytic activity. After mutagenesis with ultraviolet light, ethyl methane sulfonate or N-methyl-N'-nitro-N-nitrosoguanidine, 19,940 colonies were screened for production of enlarged zones of clearing (indicative of chitinase activity) on chitin-containing agar plates. Forty-four chitinase high producers were tested further in shake flask cultures. Mutant IMR-1E1 was isolated which, depending on medium composition, produced two to three times more than the wild type of the other components of the chitinolytic enzyme system--a factor involved in the hydrolysis of crystalline chitin and chitobiase. After induction by chitin, endochitinase and chitobiase activity appeared at similar times for both IMR-1E1 and QMB1466, suggesting possible coordinate control of these enzymes. The results are consistent with IMR-1E1 containing a regulatory mutation which increased production of the components of the chitinolytic enzyme system and/or with IMR-1E1 containing a tandem duplication of the chitinase genes. The high rate of reversion of IMR-1E1 to decreased levels of chitinase production suggests that the overproduction of chitinase by IMR-1E1 is due to a tandem gene duplication.
Full text
PDF
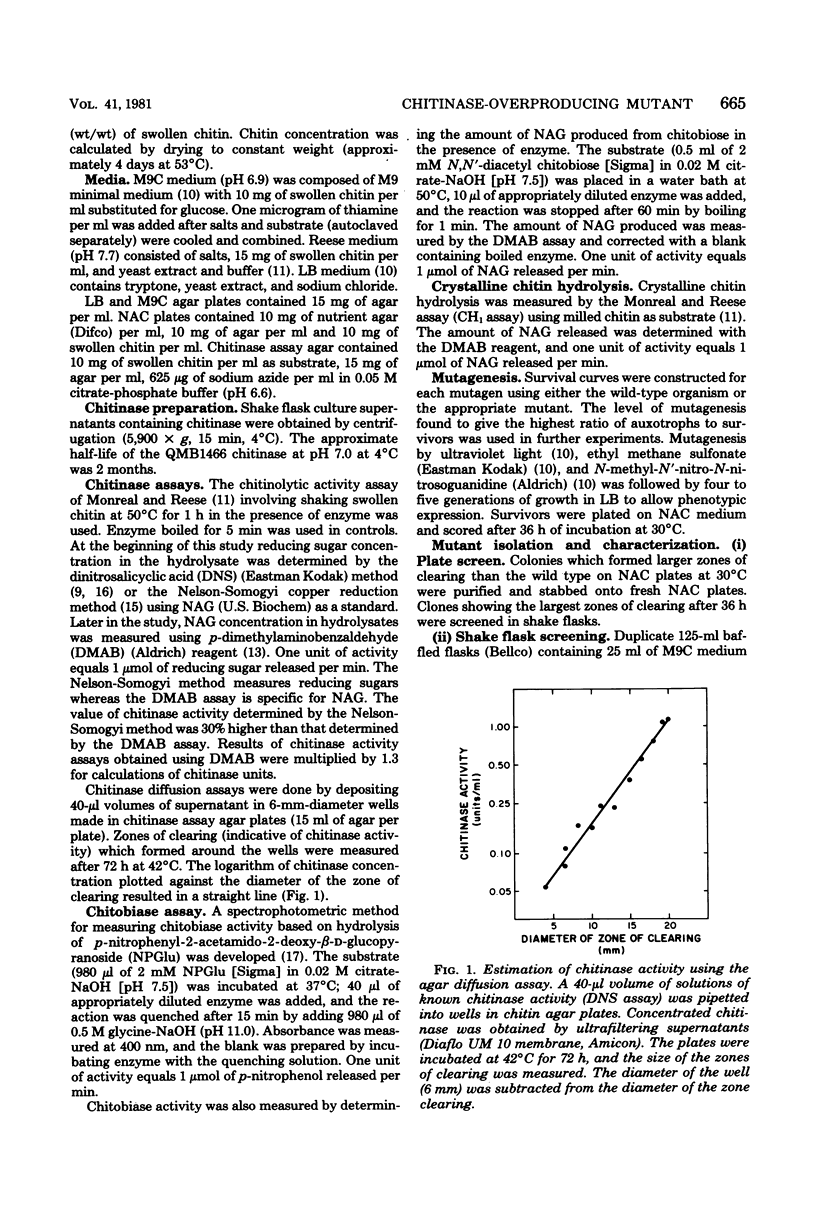

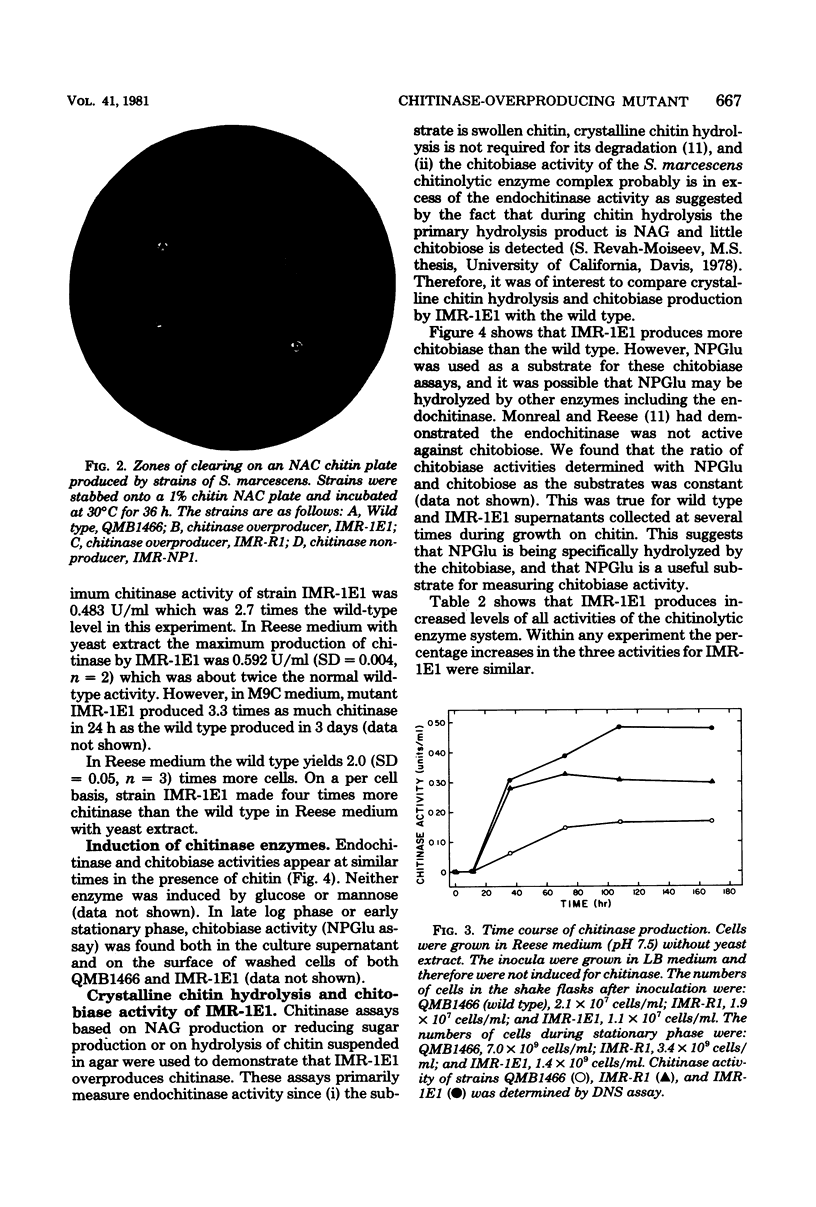
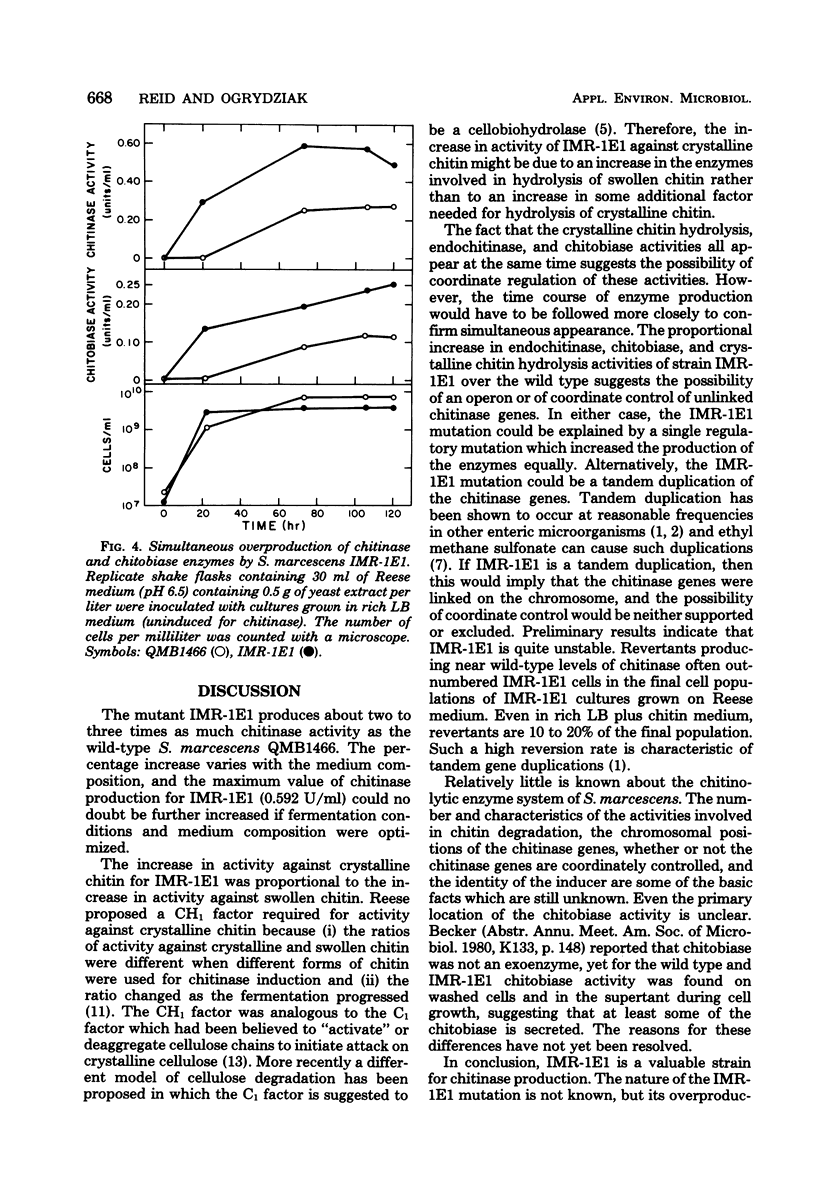
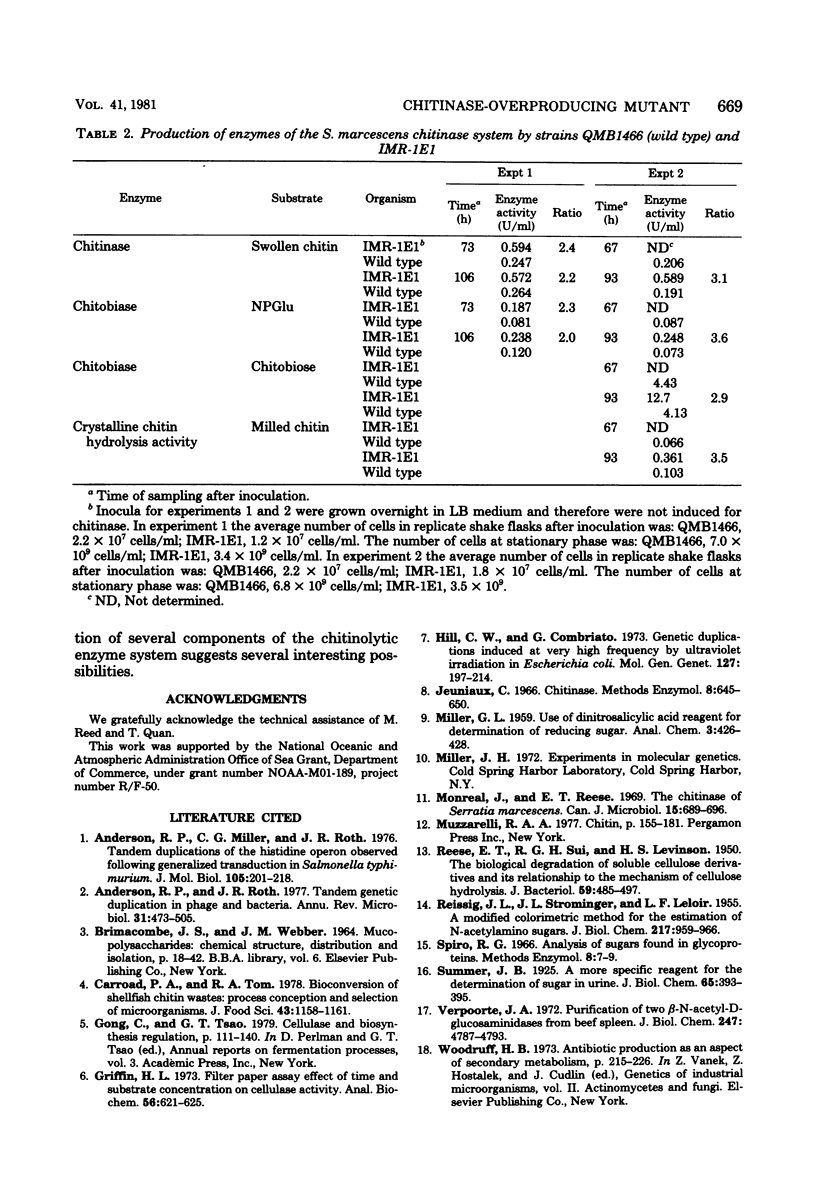
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Miller C. G., Roth J. R. Tandem duplications of the histidine operon observed following generalized transduction in Salmonella typhimurium. J Mol Biol. 1976 Aug 5;105(2):201–218. doi: 10.1016/0022-2836(76)90107-8. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Griffin H. L. Filter paper assay--effect of time and substrate concentration on cellulase activity. Anal Biochem. 1973 Dec;56(2):621–625. doi: 10.1016/0003-2697(73)90234-0. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Combriato G. Genetic duplications induced at very high frequency by ultraviolet irradiation in Escherichia coli. Mol Gen Genet. 1973 Dec 31;127(3):197–214. doi: 10.1007/BF00333760. [DOI] [PubMed] [Google Scholar]
- Monreal J., Reese E. T. The chitinase of Serratia marcescens. Can J Microbiol. 1969 Jul;15(7):689–696. doi: 10.1139/m69-122. [DOI] [PubMed] [Google Scholar]
- REESE E. T., SIU R. G. H., LEVINSON H. S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol. 1950 Apr;59(4):485–497. doi: 10.1128/jb.59.4.485-497.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Verpoorte J. A. Purification of two -N-acetyl-D-glucosaminidases from beef spleen. J Biol Chem. 1972 Aug 10;247(15):4787–4793. [PubMed] [Google Scholar]



