Abstract
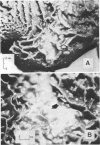
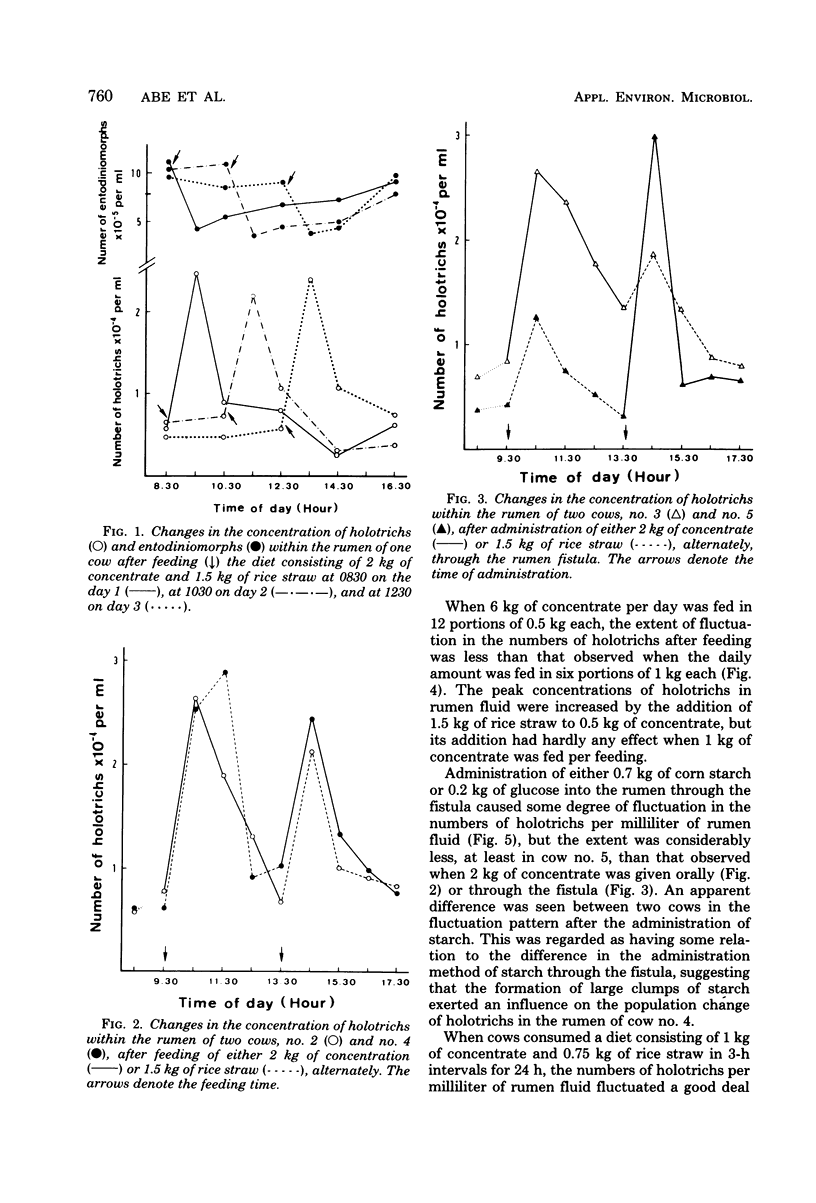
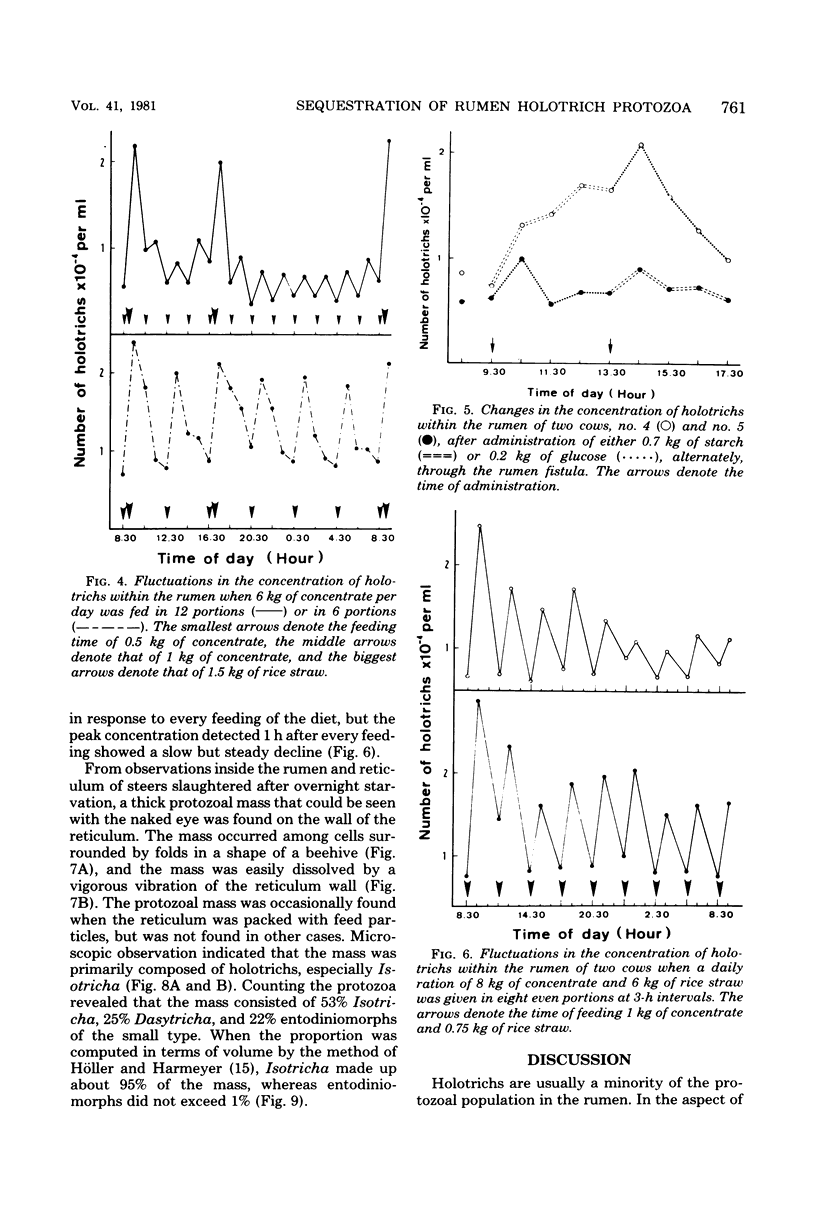
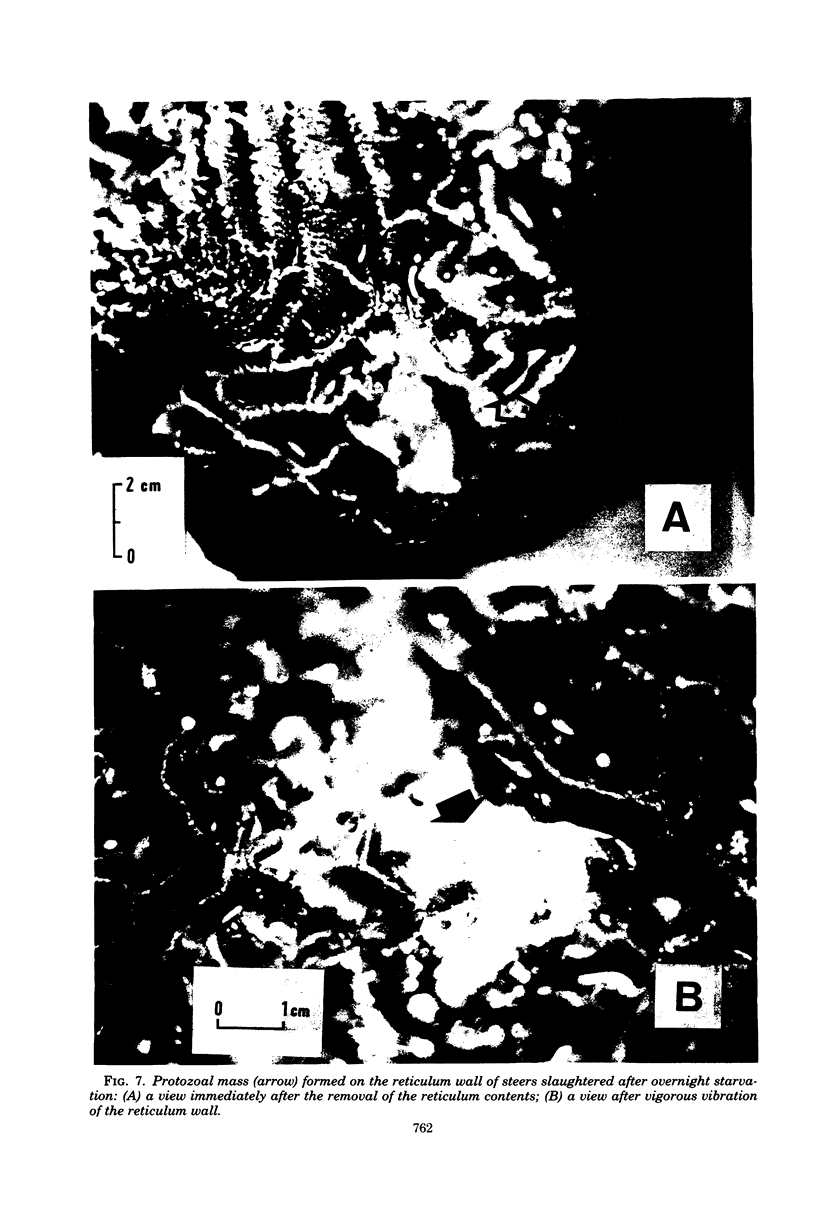
Studies were carried out to determine the means by which holotrich protozoa can maintain their numbers within the rumen against the washout effect associated with the flow of ingesta. When a diet composed of 2 kg of concentrate and 1.5 kg of rice straw was fed to Holstein cows, about a fourfold increase in holotrich numbers per ml of rumen fluid was observed within 1 h after the commencement of feeding, and an abrupt decrease followed. This fluctuation in numbers was not related to the time of feeding. A sole feeding of 2 kg of concentrate had almost the same effect on the holotrichs as a sole feeding of 1.5 kg of rice straw. Administration of either 2 kg of concentrate or 1.5 kg of rice straw through the rumen fistula caused similar changes, though the extent of response to the former was greater than that to the latter. The administration of either 0.7 kg of starch or 0.2 kg of glucose through the fistula had a relatively minor effect on the holotrich population. Addition of rice straw to 0.5 kg of concentrate increased the change in numbers, but its addition had little, if any, effect when 1 kg of concentrate was fed. These results suggested that the fluctuation in holotrich numbers was related not only to the nature or component of feed but also to other factors such as the quantity or volume of a diet and the act of ingesting feed. Increasing the number of feedings up to eight times per day at 3-h intervals caused a decrease in the peak heights of holotrich numbers per milliliter of rumen fluid. A thick protozoal mass which primarily consisted of holotrichs was found on the wall of the reticulum of Holstein steers slaughtered after overnight starvation. These findings suggest that holotrichs would usually sequester on the reticulum wall and migrate into the rumen only for a few hours after feeding, and that this mode of behavior would be essential for holotrichs to maintain their population within the rumen of cattle. Possible mechanisms of the migration are also discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Iriki T. Effects of diet on the protozoa population in permeable continuous cultures of rumen contents. Br J Nutr. 1978 Mar;39(2):255–264. doi: 10.1079/bjn19780035. [DOI] [PubMed] [Google Scholar]
- Abe M., Kumeno F. In vitro simulation of rumen fermentation: apparatus and effects of dilution rate and continuous dialysis on fermentation and protozoal population. J Anim Sci. 1973 May;36(5):941–948. doi: 10.2527/jas1973.365941x. [DOI] [PubMed] [Google Scholar]
- Abe M., Shibui H., Iriki T., Kumeno F. Relation between diet and protozoal population in the rumen. Br J Nutr. 1973 Mar;29(2):197–202. doi: 10.1079/bjn19730094. [DOI] [PubMed] [Google Scholar]
- Bauchop T., Clarke R. T. Attachment of the ciliate Epidinium Crawley to plant fragments in the sheep rumen. Appl Environ Microbiol. 1976 Sep;32(3):417–422. doi: 10.1128/aem.32.3.417-422.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird S. H., Hill M. K., Leng R. A. The effects of defaunation of the rumen on the growth of lambs on low-protein-high-energy diets. Br J Nutr. 1979 Jul;42(1):81–87. doi: 10.1079/bjn19790091. [DOI] [PubMed] [Google Scholar]
- Bird S. H., Leng R. A. The effects of defaunation of the rumen on the growth of cattle on low-protein high-energy diets. Br J Nutr. 1978 Jul;40(1):163–167. doi: 10.1079/bjn19780108. [DOI] [PubMed] [Google Scholar]
- Czerkawski J. W., Breckenridge G. Design and development of a long-term rumen simulation technique (Rusitec). Br J Nutr. 1977 Nov;38(3):371–384. doi: 10.1079/bjn19770102. [DOI] [PubMed] [Google Scholar]
- Dehority B. A., Mattos W. R. Diurnal changes and effect of ration on concentrations of the rumen ciliate Charon ventriculi. Appl Environ Microbiol. 1978 Dec;36(6):953–958. doi: 10.1128/aem.36.6.953-958.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. G., Beever D. E., Osbourn D. F. The contribution of protozoa to the protein entering the duodenum of sheep. Br J Nutr. 1979 May;41(3):521–527. doi: 10.1079/bjn19790067. [DOI] [PubMed] [Google Scholar]
- Hungate R. E., Reichl J., Prins R. Parameters of rumen fermentation in a continuously fed sheep: evidence of a microbial rumination pool. Appl Microbiol. 1971 Dec;22(6):1104–1113. doi: 10.1128/am.22.6.1104-1113.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpin C. G., Letcher A. J. Some factors controlling the attachment of the rumen holotrich protozoa Isotricha intestinalis and I. prostoma to plant particles in vitro. J Gen Microbiol. 1978 May;106(1):33–40. doi: 10.1099/00221287-106-1-33. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol. 1975 Dec;91(2):249–262. doi: 10.1099/00221287-91-2-249. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. The rumen flagellate Callimastix frontalis: does sequestration occur? J Gen Microbiol. 1974 Oct;84(2):395–398. doi: 10.1099/00221287-84-2-395. [DOI] [PubMed] [Google Scholar]
- Owens F. N., Isaacson H. R. Ruminal microbial yields: factors influencing synthesis and bypass. Fed Proc. 1977 Feb;36(2):198–202. [PubMed] [Google Scholar]
- PURSER D. B. A diurnal cycle for Holotrich protozoa of the rumen. Nature. 1961 May 27;190:831–832. doi: 10.1038/190831a0. [DOI] [PubMed] [Google Scholar]
- WARNER A. C. Some factors influencing the rumen microbial population. J Gen Microbiol. 1962 Apr;28:129–146. doi: 10.1099/00221287-28-1-129. [DOI] [PubMed] [Google Scholar]
- Warner A. C. Diurnal changes in the concentrations of micro-organisms in the rumens of sheep fed limited diets once daily. J Gen Microbiol. 1966 Nov;45(2):213–235. doi: 10.1099/00221287-45-2-213. [DOI] [PubMed] [Google Scholar]
- Weller R. A., Pilgrim A. F. Passage of protozoa and volatile fatty acids from the rumen of the sheep and from a continuous in vitro fermentation system. Br J Nutr. 1974 Sep;32(2):341–351. doi: 10.1079/bjn19740087. [DOI] [PubMed] [Google Scholar]